 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
| |
 |
In the last decades organic light-emitting diodes (OLEDs) have been extensively investigated as the potential next generation technology for flat-panel display and lighting. The interest in this technology has been triggered due to the reports of new breakthroughs in device efficiencies, lifetimes, and achievable colors, including white. However, these high performance levels are only obtained using multilayered devices. The multilayer architecture is obtained by sequentially evaporating the active species under high-vacuum conditions. Additionally, these devices use air-sensitive metals or charge-injection layers, which require rigorous encapsulation to prevent degradation. A successful entry of OLEDs into the general lighting market requires, apart from high performance levels, a significant cost reduction of the devices. In this respect, it is of particular importance to be able to generate electroluminescence from devices using air-stable charge-injection interfaces. Some examples exist; however these devices rely on the presence of ionic charges to generate a dipole across the metal–light-emitting layer interface, and their reported lifetimes are low. Metal oxides hold, in principle, the promise of good charge injection, as they combine properties such as high transparency, good electrical conductivities, tuneable morphology, and the possibility of deposition on large areas with low-cost techniques. Recently,reports concerning the use of metal oxides as charge-injection. |
| |
|
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
| |
 |
These hybrid organic-inorganic light-emitting diodes (HyLEDs) have an inverted structure compared to OLEDs; the metal oxide cathode is used as the bottom contact, on top of or instead of the conductive substrate (normally, indium- or fluorine-doped tin oxide, ITO or FTO, respectively), and a gold anode is used as the top contact. HyLEDs take advantage of the peculiar characteristics of both organic and inorganic materials. A large variety of organic semiconductors is available, and it is possible to prepare tailor-made materials. Moreover, organic thin films can be easily processed through various printing techniques, and can be deposited on flexible substrates.
HyLEDs have shown promising performance, such as high luminance levels and low turn-on voltages, but they have not displayed high efficiency, primarily due to the high current density flowing through the device. Recently, we presented the operating mechanism of HyLEDs and showed why they only work with one specific type of light-emitting polymer (LEP), poly(9,9-dioctylfluorene-co-benzothiadiazole) (F8BT) |
| |
|
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
| |
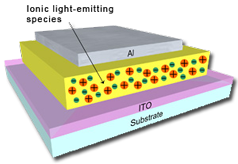 |
Solid-state light-emitting electrochemical cells (LECs) are single-component electroluminescent devices consisting of a charged luminescent material.
The main characteristic of these devices is the insensitivity to the workfunction of the electrodes employed. Therefore, in contrast to organic light-emitting diodes (OLEDs, http://www.oled-info.com/devices.html), air-stable electrodes, such as gold, silver, or aluminum can be used, which is an initial requirement for obtaining unencapsulated devices. Prototype examples of such devices were based on conjugated polymers to which inorganic salts were added. More recently, single-component, solid-state light-emitting devices based on ionic transition-metal complexes (iTMCs) have been reported. In iTMC-based LECs, the ionic complexes perform all the necessary roles for the generation of light: a) the lowering of the injection barrier by the displacement of ions, b) the transport of electrons and holes by consecutive reduction and oxidation, respectively, of the iTMC, and c) the generation of the photons by phosphorescence |
| |
|
 |
 |
 |
 |
|
| |
|
|
 |