Solutions 1
The following figure shows a sample of solid Na2SO4 ready to be dissolved in water.
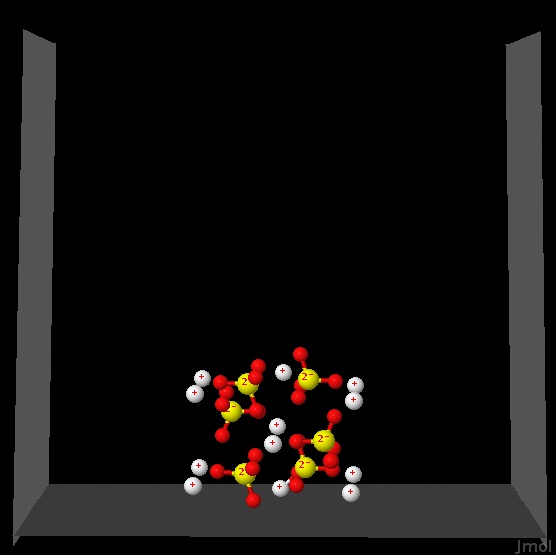 |
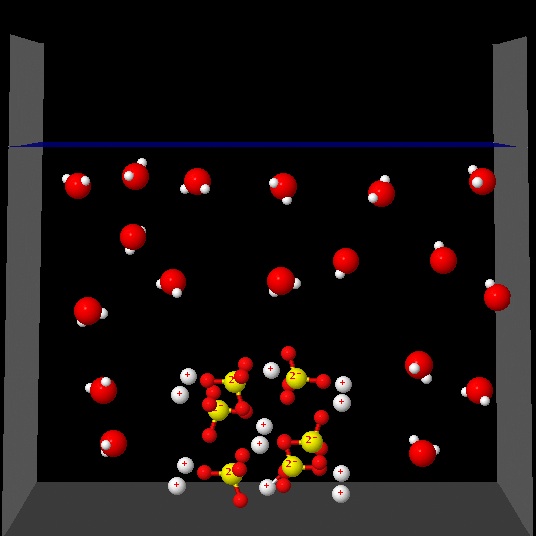
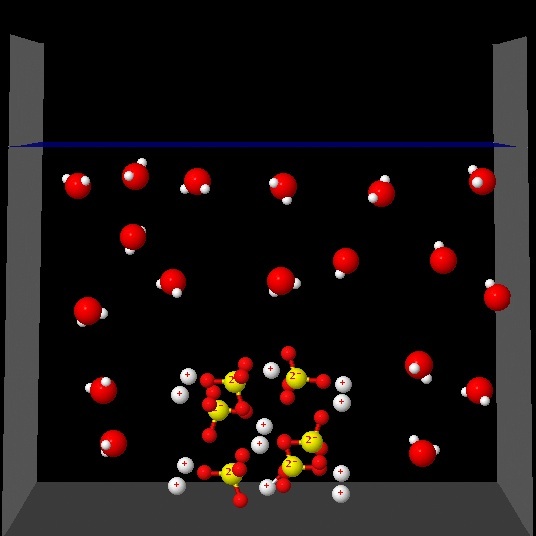
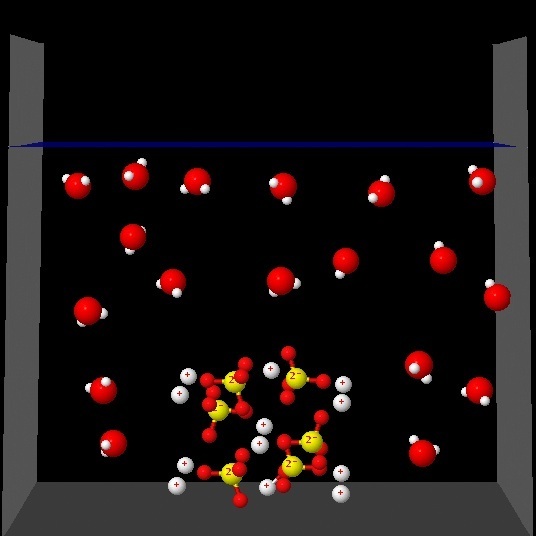
Click on each of the following figures and observe an animation
 |
 |
 |
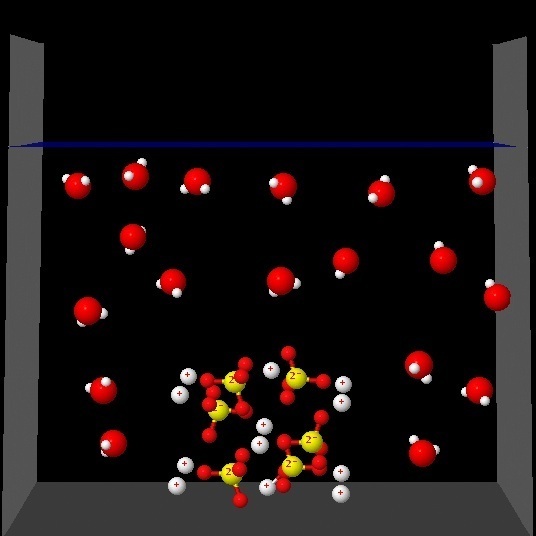 |
Which one best represents the changes that occur in the solution process?.
Correct. In the presence of water the ions are distributed regularly, surrounded by water molecules. Ion-dipole (water) interactions are not shown.
|
|
Wrong. This animation shows the water molecules
around the solid sample without separation of ions in the aqueous medium.
|
|
Wrong. The animation shows that ions are separated but not water molecules around the ions.
|
|
Wrong. The animation shows that ions are separated from the solid but left in the bottom of the glass. No water molecules around the ions.
|