SALSOLCHEMIS: a spreadsheet application to calculate the ionic speciation of saline soil solutions and irrigation waters
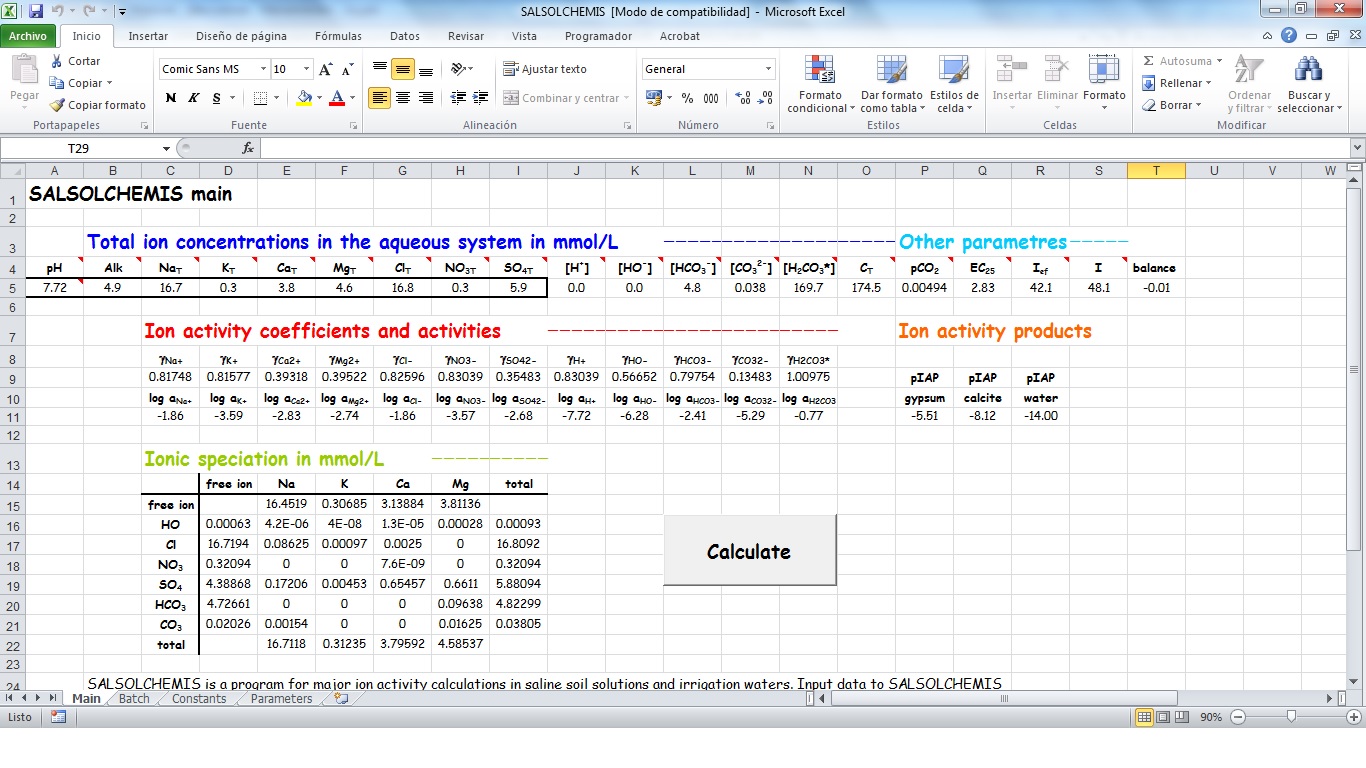
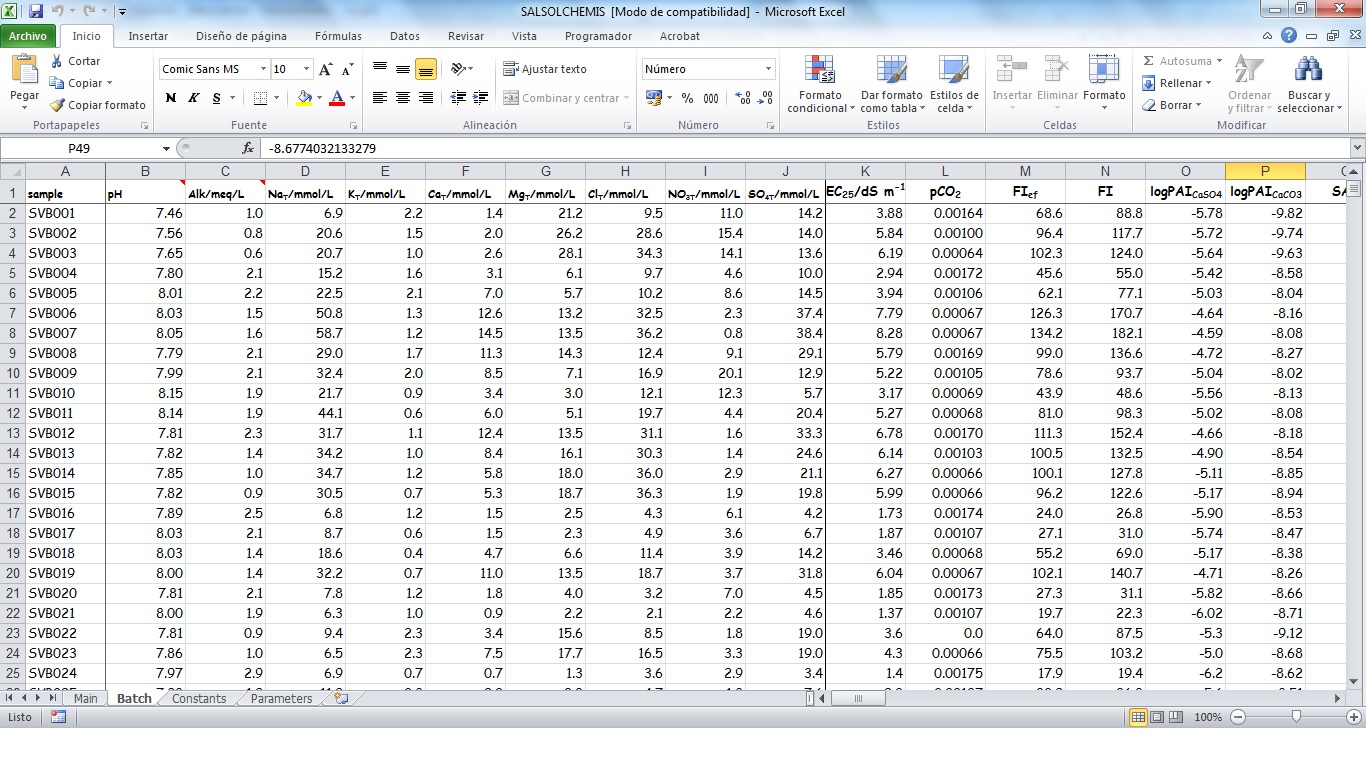
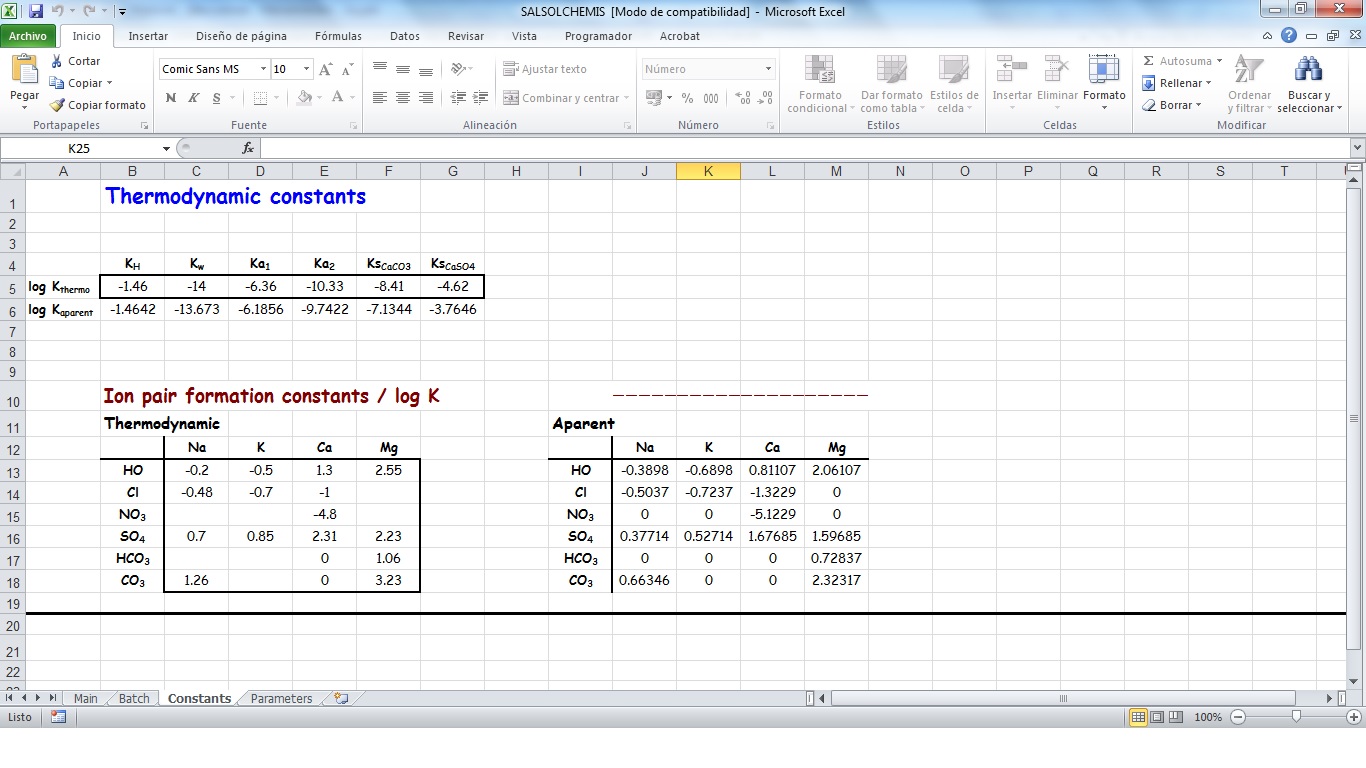
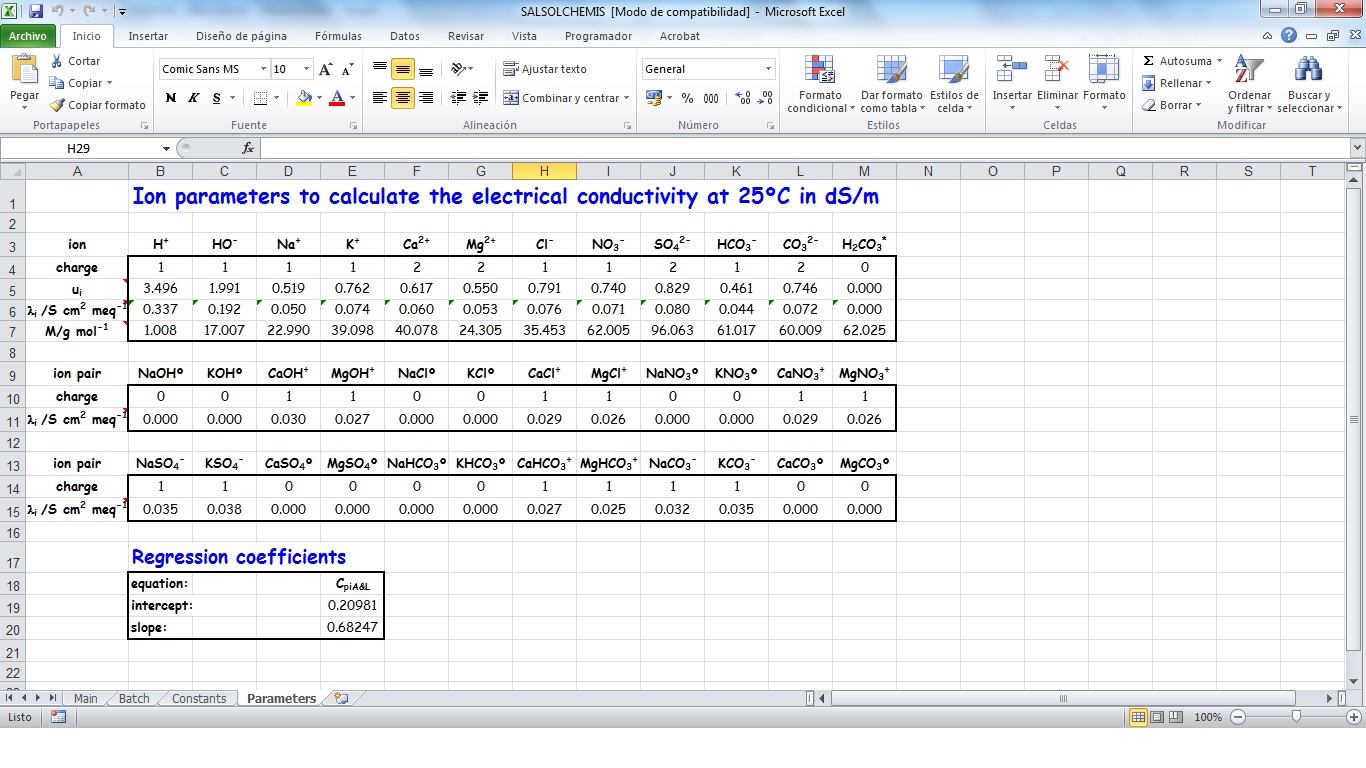
SALSOLCHEMIS (SALine SOLution CHEMistry Ionic Speciation) is a computer program developed to calculate the equilibrium properties of saline, calcareous and gypsiferous soil solutions and irrigation waters at 25ºC, pH between 3 and 11 and electrical conductivity (EC25) lower than 20 dS m-1. SALSOLCHEMIS is an ion-association model for the ions sodium, potassium, magnesium, calcium, chloride, nitrate, sulphate, bicarbonate and carbonate. SALSOLCHEMIS is able to carry out calculations of ionic speciation, ionic activities, ionic activity products, and EC25. SALSOLCHEMIS is written in Microsoft Visual Basic© and developed as a spreadsheet application. Input data to the model are entered in the sheets of a Microsoft Excel© workbook, from which SALSOLCHEMIS is called. Following calculations output data are written in the same workbook.
The essential steps for running the SALTIRSOIL model are described below.
SALSOLCHEMIS is copyrighted software by the author, distributed free for educational and research purposes and downloadable here.
The SALTIRSOIL_M model has featured several research articles which can be accessed from this page.