| vegetation measurements |
This page offers a description of the vegetation instruments and measurements available during the campaign.
INSTRUMENTATION
A large amount of ground measurements will be collected in the Barrax study area during the SEN2FLEX campaign, covering LAI, fCover, Leaf Chlorophyll a+b, Leaf water content and leaf biomass, together with other complementary data. All the available ground measurements will be cross-checked with GPS measurements.
The sampling strategy to be followed in choosing measurement points has been designed according to statistical criteria. Nevertheless, most times experimental constraints become critical, and finding a balance between what is mathematically optimal and what is experimentally possible requires some compromise.
According to statistical requirements, till 4 to 15 areas have to be sampled to fully characterize a crop (its size and heterogeneity). These areas will be chosen randomly, but it is not operationally very often (high plant densities, very wet soils or tall plants may cause moving across the cross be very hard). So, the aim is to perform random sampling in crops where it will be possible, but restrict sampling to crop geometrical constraints when it will be not. For example, taking samples in 1.80 meters high corn crops following the circular track made by irrigation pivot wheel could be a good idea.
Once sampling areas wil be chosen, a strategy to take samples inside each area will be again needed. The above considerations on the choice of areas will be again useful in point sampling within the sampling area: random criterion is mathematically supported, so it will be the one to be followed when field crop configuration allows it; however, it will be sometimes difficult to move around one measurement point to take samples randomly, so mobility requirements shall be taken into account in such cases, and a sampling strategy that made sampling easier shall be be followed. Biophysical parameters that will be used in the characterization of the different crops:
a. Dry Matter content (DM)
b. Water Content (WC)
c. Leaf Area Index (LAI) from LAI-Licor
d. Fractional Vegetation Cover (FVC) from hemispherical photographs
e. Chlorophyll Content (CC)
• LI-COR LAI-2000 instrument
The LAI-2000 (Li-Cor inc., Lincoln, NE, in fig. 6.1) uses a fish-eye lens with an hemispheric field of view (?148º). The detector is composed of five concentric rings (sensitive to radiation below 490nm). Each ring responds over a different range of zenith angles. Light attenuation through the canopy is measured and light transmittance is calculated as the ratio of below: above canopy readings for each of the 5 angles. This ratio is used to calculate several variables, including leaf area index, gap fraction, and mean tilt angle (a number representing the foliage orientation).

Figure 6.1 LAI2000 Plant Canopy Analyser
• GARMIN´s GPS 12 cx color personal navigatortm
The Garmin GPS 12CX color-display Personal Navigator (figure 5.1) provides satellite navigation you can count on.
- Receiver: 12 parallel channel receiver continuously tracks and uses up to twelve satellites to compute and update a position
- Acquisition Times:
o Warm: approx. 15 seconds
o Cold: approx. 45 seconds
o AutolocateTM: approx. 5 minutes
o Ezinit: aprox 45 seconds
o Update Rate: 1/second, continous - Accuracy:
o Position: 15 meters RMS. The RMS is subject to accuracy degradation to 100m 2DRMS under the United States Department of Defense-imposed Selective Availability Program.

Figure 6.2. GARMIN´s GPS 12CX
• Digital camera NIKON Coolpix5000 with a NIKON FC-E8 fisheye
converter
Hemispherical canopy photography is a technique for studying plant canopies via photographs acquired through a hemispherical (fisheye) lens from beneath the canopy (oriented towards zenith) or placed above the canopy for downwards looking. Therefore, it can be used for any canopy type.
A hemispherical photograph allows you to derive, among others, vegetation structural characteristics such as, gap fraction, leaf area index (LAI) and average leaf inclination angle (ALA). It provides a permanent record and is therefore a valuable information source for position, size, density, and distribution of canopy gaps. The view angle is equal to 183º and allows the gap fraction to be evaluated in all viewing directions, which increases the accuracy of the derived biophysical variables (LAI, ALA).

Fig 6.3. NIKON Coolpix5000 with the NIKON FC-E8 fisheye converter.
Time-Domain Reflectometry (TDR)
The main components of a typical TDR instrumentation are schematically drawn in figure 6.4. The electronic apparatus includes a control unit, needed for the synchronisation of the pulse generator and the receiver, and the output device (generally a LCD monitor) and related peripherals (communication port and/or dot matrix printer). The apparatus is connected with a coaxial cable to the transmission line (TDR probe), which may have different shapes and configurations.

Figure 6.4. Schematic view of TDR apparatus (adapted from Topp and Davis, 1985)

Figure 6.5. Example of TDR output with a balanced parallel line in a wetted soil.
The returning signals are superimposed to the emitted ones and they are detected by the receiving unit, with a voltage decrease or increase depending on the phase of the reflection. By means of its built-in hardware and firmware, the receiving unit samples and records the returning signal at very small time steps; in the same time, the output device provides a graph of the reflected voltage versus time.

Figure 6.6. Balanced probes of different length.
A widely used TDR equipment is the Tektronix cable tester Mod.1502 C, which has been conceived as a portable measuring device and it can be easily connected to a personal computer through its communication port. In this case, the step-pulse is characterised by an amplitude of 300 mV, a rise time of 0.2 ns and a duration of 25 ms corresponding to a theoretical frequency range from 20 kHz and 1.75 GHz. The effective frequency bandwidth is certainly restricted by attenuation effects induced by cable connections; the extent of this attenuation, which mainly affects the higher frequencies range, largely depends on the quality and on the length of cables. From the analysis of several estimates of soil dielectric permittivity on different types of soils by means of TDR, Heimovaara et al. (1994) estimated a frequency range between 200 MHz and 1 GHz, thus confirming the assumptions reported in previous section.

Figure 6.7. Parallel balanced
(a) and unbalanced (b) TDR probes (from Whalley, 1993)
The probe inserted into the soil constitutes the pulse transmission line which can be electrically balanced or unbalanced. The balanced lines (figure 6.6) are more widely used and they are made of parallel and cylindrical metallic conductors; the soil between them is the dielectric medium (figure 6.7-a). The probe is connected to the TDR unit by means of a coaxial cable or a parallel shielded cable; every connection represents an impedance mismatching which causes a partial reflection of energy.

Figure 6.8. Unbalanced three-wire probe (L=14 cm) with coaxial cable and connector.






Chlorophyll Content Meter
The New CCM-200 Chlorophyll Content Meter (fig. 6.4.) from Opti-Sciences
accurately determines chlorophyll content in plants and crops. Especially
useful for improving Nitrogen management programs, the CCM-200 is also
an ideal instrument for research and teaching. The CCM-200 provides agronomists,
researchers, and teachers with reliable, repeatable chlorophyll content
readings. The ample on-board data storage and hand-held design make the
CCM-200 the most field efficient, affordable chlorophyll content meter
on the market.
Changes in chlorophyll content can occur as a result of nutrient deficiencies,
exposure to environmental stress, exposure to certain herbicides, and
differences in light environment during growth (shading). Chlorophyll
content can be used to manage nutrient optimization programs that both
improve crop yield and help protect the environment. Testing for herbicide
damage can indicate the need for a change in herbicide selection or application
methods; in order to maintain good weed control while having minimum impact
on crop health.
Observation of changes in chlorophyll content have applications in basic
photosynthesis research. The CCM-200 illustrates changes in chlorophyll
content which can be correlated to plant health and condition. This data
can even be used to compliment chlorophyll fluorescence and gas analysis
measurements.
Laboratory methods for determination of chlorophyll content are both time
consuming and destructive to the sample. Typically a sample must be detached,
ground up in a solvent, then assayed in a spectrophotometer. A sample
can be measured only once precluding the monitoring of trends in chlorophyll
content over the growing cycle. The CCM-200 provides non-destructive,
rapid measurements of relative chlorophyll content without the need to
detach and grind a sample.
Chlorophyll has several distinct optical absorbance characteristics that
the CCM-200 exploits to measure relative chlorophyll concentration without
destructive sampling. Strong absorbance bands are present in the blue
and red but not in the green or infrared bands, hence the green appearance
of a leaf. By measuring the amount of energy absorbed in the red band
an estimate of the amount of chlorophyll present in the tissue is possible.
Measurements in the infrared band show absorbances due to cellular structure
materials. By using this infrared band to quantify bulk leaf absorbance,
factors such as leaf thickness can be taken into account in the CCI (Chlorophyll
Content Index) value.

Fig. 6.4 Chlorophyll Content Meter CCM-200
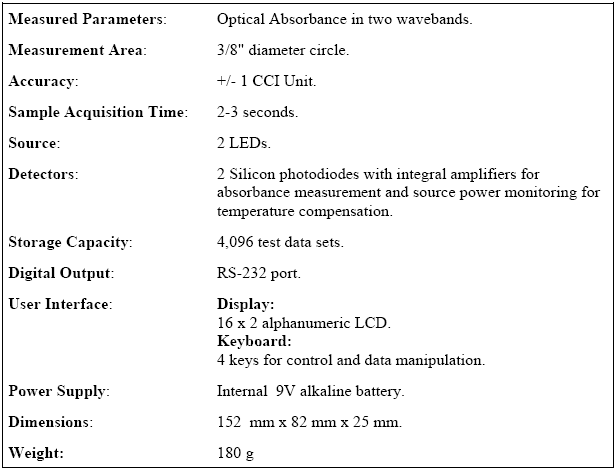
Table 6.1- CCM-200 Technical Specifications
Chlorophyll Meter SPAD-502
The SPAD-502 is a compact meter designed to help users improve crop quality and increase crop yeild by providing an indication of the amount of chlorophyll present in plant leaves.
|
SPECIFICATIONS |
|
| Type |
Handheld meter for measuring chlorophyll using optical density difference at two wavelengths |
| Measurement Sample |
Crop leaves |
| Measurement System |
Optical density difference at two wavelengths |
| Measurement Area |
2 x 3 mm |
| Light Source |
2 LED's (light emitting diodes) |
| Receptor |
1 SPD (sillicon photodiode) |
| Display |
Measurement
data: 3 digits LCD with decimal point |
| Data Memory |
Space for 30 data sets |
| Controls |
Power switch, average key, all data delete key, one data delete key, and data recall key |
| Power Source |
2 AA size alkaline manganese (1.5V) batteries |
| Battery Life |
More than 20,000 measurements |
| Min. Interval Between Measurements |
Less than 2 seconds |
| Accuracy |
With in +/-1.0 SPAD unit (at room conditions, SPAD value between 0 and 50) |
| Repeatability |
Within +/-0.3 SPAD units (SPAD value between 0 and 50) |
| Reproducibility |
Within +/- 0.5 SPAD units (SPAD value between 0 and 50) |
| Temp. Drift |
Less than +/-0.4 SPAD units/°C |
| Temp. Range |
Operation:
0 to 50°C |
| Dimensions |
14 x 78 x 49 mm (6-7/16 x 3-1/16 x 1-15/16 in.) |
| Weight |
225g not including batteries |
| Other |
Warning
buzzer |

Figure 6.10 Chlorophyll Meter SPAD-502
GPS Trimble Pathfinder Pro XRS
Versatile GPS receiver that offers submeter accuracy in real time, and centimeter level postprocessed accuracy. It is used with TSCe controller, rugged and adaptable handheld data collector that provides exceptional control of Trimble GPS and optical sensors.

Key features:
- Incorporates a 12-channel GPS receiver, differential beacon, and satellite differential receiver in one housing
- Receives SBAS real-time corrections
- Uses patented EVEREST™ multipath rejection technology to remove multipath signals in reflective environments
- Supports NMEA-0183 output and RTCM SC-104 input/output
Chlorophyll Measurements





MEASUREMENT PROTOCOLS
Crop phenology
A phenological state is the growth stage during which the plants exhibit particular physiological and/or morphological characteristics. It can be determined by comparing the plant morphology to stages defined for that crop (figure 6.11). The stage in which is the 50% of the plants is usually assumed.


Figure 6.5 Standard crop phenology scale, for cereals and legumes.
Existing procedures for growth stage description which are available in
the agronomic literature should be used. These vary between crops and
sometimes between countries or research groups. Growth stage descriptions
developed for remote sensing studies are also available. The system actually
employed should be referenced or presented in full. A simplification of
an existing system is permissible.
The minimum sample size is one in case of a uniform stand. The identification of different growth stages and the associated proportions of the canopy are important especially for larger, heterogeneous areas and at certain growth stages when the canopy changes very rapidly.
Green vegetation cover fraction
It will be evaluated from a digital picture, taken from the vertical
direction and at a constant height, with the Sun in such a position that
shadows proportion is minimal. In order to obtain the green vegetation
cover fraction a classification of the registered image will be performed.
Wet and dry biomass
This is the mass of plant material within a defined area divided by the area size (kg•m-2). For taking the measurements, cut all of the plants from a pre-defined area. If applicable, separate plants into components (stems, leaves, fruits) and place into separate containers (plastic, bags, etc.). Weigh each component within a few hours (preliminary test can be used to determine permissible elapsed time). Dry the plants at 70ºC until constant weight is reached and weigh again. From the two masses and the known sampled area, wet and dry biomass can be calculated. Water content is calculated as the percentage of wet (or dry) mass, or per unit area (volume) when biomass (biomass and height) are known.
Sample size depends on the size of the individual plants and available
drying facilities. A minimum of 150 grams of matter for small crops or
plant components should be in one sample. The minimum number of samples
is five.
Water content
This parameter is the mass of water in a plant sample divided by the mass of the entire plant sample before drying (i.e. on a wet biomass basis). Units are percent or dimensionless, although when plant height and/or biomass are known, water content can also be expressed in kg/m3 or kg/m2, respectively). It is given from the biomass evaluation procedure directly, for each one of plant components.
Leaf Area Index
It will be calculated by means of the LICOR 3000 device in the laboratory, from destructive samples. Comparative measurements will be done with other systems in the field (SUNSCAN, TRAC, LICOR 2000, hemispherical camera…), in order to validate those from the laboratory.
Leaf Chlorophyll
The leaf chlorophyll content will be measured with the CCM-200 Chlorophyll Content Meter (figure 6.4). However, it performs relative measurements, so calibration measurements must be made before using laboratory analysis methods. Leaf disks will be cut with a calibrated cork borer, wrapped in aluminum foil, frozen in liquid-nitrogen, and stored (still wrapped in foil) at -20 °C. Leaf pigments will be later extracted with acetone in the presence of Na ascorbate and stored as described previously (Abadía and Abadía 1993). Pigment extracts will be thawed on ice, filtered through a 0.45 µm filter and analyzed by an isocratic HPLC method based on that developed by De las Rivas et al. (1989). Two steps (instead of three) will be used: mobile phase A (acetonitrile:methanol,7:1,v:v) will be pumped for 3.5 min, and then mobile phase B (acetonitrile :methanol: water: ethyl acetate, 7:0.96:0.04:8 by volume) will be pumped for 4.5 min. To both solvents 0.7% (v:v) of the modifier triethylamine (TEA) will be added (Hill and Kind 1993) to improve pigment stability during separation. All chemicals used will be HPLC quality. The column will be equilibrated before injecting each sample by flushing with mobile phase A for 5 min. The analysis time for each sample will be 13 min, including equilibration time.
Plant density
This is the number of plant stems per unit area. For row crops, the method consists in counting the number of stems along the row and converting it into density using the average row width. For randomly distributed plants, count the number of stems within a predefined area. One or several stems may be originated from a root system of one plant.
Sample size varies with the crop. The area included in one sample should be large enough to contain a minimum of 30 (for large, e.g. corn) or 60 (smaller crops, e.g. cereals) stems. For rice, a sample area of 25x25 cm can be sufficient. A minimum of three samples should be taken in a homogeneous canopy, but the number should be increased for heterogeneous canopies and/or for larger areas.
Plant height
This is the distance between the canopy component of interest and the soil surface for non flooded fields. For the measurement, it must be placed a measuring stick near selected plants. Take a sufficient number of measurements to obtain a representative value. Several height parameters are useful; in the general order of decreasing importance they are:
• Total maximum height (to the top of the canopy).
• Height from the ground to the highest green leaf.
• Fruit length and diameter.
• Height from the ground to the lowest green leaf.
• Distance between leaves along the stem (internodal distance).
• Stem diameter near the ground.
A minimum of five measurements is recommended for relativity uniform canopies. This number should be increased as canopy variability and/or the area measured increase.
Plant row direction
It can be defined as the orientation of the plant rows, given in degrees or geographic direction. It must be identified the row direction of the plants, if any, and relate it to the North direction. This can be done in situ or from aerial photographs with a scale large enough, so that the row direction can be recognized. Note that plant row direction may not coincide with tillage direction.
Only one observation is needed if the row direction remains constant. However, it is important to ascertain that this is the case, as FOW direction may vary due to the field shape, cultivation pattern and natural obstacles (topography, woody, vegetation, etc…). In these instances, aerial photographs are the preferred method.
Canopy structure
This is the three dimensional location and orientation of plants and plant components in the canopy. A direct measurement would involve a reconstruction of the three-dimensional distribution of the canopy. Although very desirable, not rapid and effective method has been developed yet.
The following approach can be used to characterize the leaf distribution: leaf position is determined by placing a gridded horizontal plate beneath the canopy (x, y coordinates) and by measuring its height (z) above the plate; leaf inclination angle (in degrees) is measured with an inclinometer; leaf azimuth angle is determined (for 45º increments) using a small plate equipped with a circular bubble level and a dowel attached perpendicularly to the face of the plate. To measure the relevant physical dimensions of each canopy component (leaves, stems, heads), one should first select an appropriate geometric model for that component (e.g., cylinder, ellipsoidal disk, plate) and then measure the length, width, diameter or axes as appropriate for the assumed model (for stems, diameters at bottom and top should be measured). Among the numerous parameters describing canopy structure, two types deserve special attention: leaf dimensions and the orientation of leaves and stems. It is also helpful to Xerox plant components for a permanent record.
For a qualitative characterization of the canopy, photographs can be taken showing the vertical profile of one plant or one group of plants, preferably with a gridded background plate put vertically behind a plant row.
Soil moisture
It is defined as the amount of water held in a unit mass (gravimetric basis) of soil. The weight of water in a soil sample is divided by the weight of the sample after drying. Units are g•g-1 (gravimetric basis). Bulk density should also be determined where gravimetric samples are taken.
Although various methods for measuring soil water content are available, the destructive sampling method has been widely used because of its simplicity. A sample of soil is taken from a layer of the soil profile to be characterized. This can be accomplished using a spatula for gravimetric moisture content.
The moist samples should be weighed as soon as possible and then dried at 105ºC to a constant weight (generally 24 hours or less). Alternatively, drying in a microwave oven can produce good results and take much less time (less than 1 hour depending on sample size); a preliminary test should be carried out to calibrate this method of drying against the above standard. Using wet and dry soil masses and the volume (if known) water content values can be calculated.
It is recommended for ASAR/ENVISAT that the layer 0 to 5 cm in depth always be sampled and reported to ensure comparability of results among different studies. To obtain representative mean values, it is recommended as a general guideline that 5 or more (minimum 3) individual samples be taken.
Soil roughness
Soil roughness is the shape of the soil-air interface in three dimensions. The roughness components are clods or soil aggregates random, furrows or rows (periodic), and slope or inclination of the surface (monotonic). In practice, it can be described by the spatial correlation function of surface roughness in two perpendicular directions.
Optimally, the measurements technique should produce data with horizontal and vertical resolutions of one-tenth of the radar wavelength studied. In addition, measurements should be made over a consecutive length of up to 10 times the largest roughness periodicity (e.g. across 10 rows of a crop). For distinct periodic surfaces (e.g. plowed) these measurements should provide slope angle distribution as well.
Some methods are photographs of a gridded metal panel inserted into the soil and levelled which are subsequently digitized, water resistant paper attached to the metal plate and the cross section sprayed with a colour spray paint. It is important that surface roughness is not changed during the panel insertion or spraying. This is impossible to achieve under some conditions, e.g. for a loose, dry soil where this approach should be avoided.
VEGETATION MEASUREMENTS PLANNING
Sampling for each crop is currently being defined, as well as the measurements that must be made simultaneous with satellite/aircraft overpasses (chlorophyll and water, surely) and which not (LAI and FCV). Figure 6.12 shows the RGB LANDSAT-5 image composition reported by IDR corresponding to may-26 of 2005 overlaped to the INTA and DLR flights configurations planned to Mission 1 of SEN2FLEX campaign.

Program of activities planned for the SEN2FLEX campaign
A monitoring system for soil water content, based on a TDR system (Tektronix) that will be operating continuously from June 2nd to July 18th, will be installed. Randomly distributed soil water content measurements will also be performed during the flights. In addition, it will be performed some LAI measurements in selected points (for validation only) and if needed spectro-radiometer measurements.
| Date |
Activity |
Measurements |
| 28 May- 3 June: Installation of TDR system
Operating continuously from June 2nd to July 18th |
Installation of a monitoring system for soil water content based on a TDR system (Tektronix) with a multiplexer connected to 20 probes. TDR measurements (31 May - 2 june) |
The installation field should also be equipped with one flux tower measurement. A second similar field with Enviroscan probes could be set up by UCLM-IDR on an alfalfa field. We will have two locations for a multitemporal validation of a water flow model and a energy balance model.
|
|
From May 31th to June 3th |
Randomly distributed soil water content measurements will also be performed during the flights
|
Soil water content measurements will also be performed during the flights |
|
From May 31th to June 3th |
Some LAI measurements in selected points (for validation only) and if needed spectro-radiometer measurements.
|
For validation : LAI and spectro-radiometer measurements (if needed) in selected points. |
|
20-24 June: 2nd visit of electronic engineer for checking TDR station and data download
|
Visit of electronic engineer for checking TDR station and data download
|
TDR station checking and data download
|
|
10 -16 July
|
Full team in Barrax for Mission 2 of SEN2FLEX activities
|
To follow the same strategy that during Mission-1 |
UNIVERSITY OF VALENCIA
Characterisation of the biophysical parameters that will be used in the characterization of the different crops:
a. Dry Matter content (DM)
b. Water Content (WC)
c. Leaf Area Index (LAI) from LAI-Licor
d. Fractional Vegetation Cover (FVC) from hemispherical photographs
e. Chlorophyll Content (CC)
| Date |
Sample |
Measurements |
|
01/06/2005 |
|
· Along the day: LAI measurements. · 12-14 H.: Chlorophyll content sampling with the CCM-200 and the SPAD-502 to intercompare instruments and measurements. · 5-7 P.M: LAI and FVC measurements. |
|
02/06/2005 |
|
Characterization of the different canopies present in the Las Tiesas area: · 6-8 A.M: LAI measurements. · 10-12 A.M: Biomass and water content sampling to characterice vegetation at study area.12-14 H.: Chlorophyll content sampling with the CCM-200 and the SPAD-502 to intercompare instruments and measurements. · 5-7 P.M: LAI and FVC measurements. |
|
03/06/2005 |
|
Characterization of the different canopies present in the Las Tiesas area: · 6-8 A.M: LAI measurements. · 10-12 A.M: Biomass and water content sampling to characterice vegetation at study area.12-14 H.: Chlorophyll content sampling with the CCM-200 and the SPAD-502 to intercompare instruments and measurements. · 5-7 P.M: LAI and FVC measurements. |

Figure 6.13. Vegetation sampling for the SEN2FLEX campaign Mission-1 overlaped to the RGB LANDSAT-5 image composition reported by IDR corresponding to may-26 of 2005.