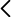Ron Geller, researcher of the Institute of Integrative Systems Biology (I2SysBio) mixed centre of the Universitat de València and CSIC, in collaboration with American research groups, has demonstrated that the chaperone protein Hsp90 has an impact on other proteins. The chaperone protein help others to wrinkle, showing which mutations are tolerated in their consequences. The work has studied the protein evolution in the poliomyelitis virus and it explains the procedures through new mutations.
The experiment has been yesterday published in the journal Nature Communications, in which the chaperone protein Hsp90 has been used. As functions, it helps other proteins to fold adequately, to stabilize them in situations of hyperthermia, to help in the degradation of other proteins and also to stabilize other involved in the growth of some tumours. For this reason, the inhibitors of the Hsp90 are investigated as medicines against cancer.
The study has identified how chaperone proteins affect in the evolution of proteins that help to fold, the question that was uncertain until now. The experiment shows for the first time how chaperone affect to the general evolution of some proteins in a specific form. They help to choice some kind of specific mutations, but however, refusing others.
Research group has selected the poliomyelitis virus under consideration to observe the response of its proteins to the effects of Hsp90. This virus has only eleven proteins, and also has a great evolving capacity, a short period of replication (eight hours per cycle), a high rate of mutation and a large number of population (1.000 millions per millilitre).
In the research team are Ron Geller, of the Institute of Integrative Systems Biology; Sebastian Pechmann, of the Montreal University; Ashley Acevedo, of the Rockefeller University of New York; Raul Andino, of the San Francisco University; and Judith Frydman, of the Stanford University.
This research was funded by the Ministry of Economy, Industry and Competitiveness (MINECO), through Ramón y Cajal support and a grant awarded to Judith Frydman by the National Institute of Allergy and Infectious Diseases (NIH) of United States.
Complex biological systems
The Institute of Integrative Systems Biology (I2SysBio), mixed centre of Universitat de València-CSIC, study the complex biological systems, especially micro-organism, with applications mainly in biomedicine and biotechnology. The centre operates through an innovative model of public-private research. It is located in the Science Park of the Universitat de València, in the Burjassot-Paterna Campus.
Article:
Ron Geller, Sebastian Pechmann, Ashley Acevedo, Raul Andino & Judith Frydman: «Hsp90 shapes protein and RNA evolution to balance trade-offs between protein stability and aggregation». Nature Communications volume 9, article number: 1781, (2018) doi: 10.1038/s41467-018-04203-x
Caption:
Examples of mutations enriched with the poliovirus protein in normal conditions (blue) or Hsp90 (red)
Images: