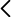Research Group: Bacterial Pathogenomics
Tuberculosis remains the first cause of adult death by a single infectious agent worldwide, despite a century of research leading to valuable tools to reduce tuberculosis mortality. Therefore, innovative approaches are needed to palliate the dramatic burden of tuberculosis damage on mankind. Our knowledge of the interplay between bacterial virulence and human immunity remains insufficient in the context of tuberculosis. I therefore propose to explore host-pathogen associations as a mean to decipher yet unknown mechanisms underlying tuberculosis in Africa.
Tuberculosis is caused by different members of the Mycobacterium tuberculosis complex (MTBC), that includes M. tuberculosis sensu strictu (Lineages 1 to lineage 4 and Lineage 7) and M. africanum (Lineage 5 and Lineage 6). The distribution of tuberculosis worldwide disproportionately affects Africa, Asia, and Eastern Europe, while Western Europe, the USA and Canada, and Oceania experience far lower rates of disease. Among the whole MTBC, M. africanum (Lineage 5 and Lineage 6) causes up to half of human tuberculosis in West Africa. However, research in M. africanum is still scarce, and genomic studies are particularly infrequent.
M. africanum is metabolically and clinically different from other MTBC lineages, and it is almost restricted to West African countries. Although M. africanum has been sporadically found in epidemiological studies outside West Africa, tuberculosis patients carrying M. africanum were mostly immigrants from West Africa. The reason why M. africanum is still restricted to West African populations despite old and modern human migrations out of Africa remains unknown.
Considering the long-term association between MTBC and its human host, some degree of co-evolution is likely to have occurred. Indeed, several recent studies have shown that the susceptibility to TB is influenced by variation in both the pathogen and the host. Hence, the interactions between host and pathogen genomic diversity needs to be explored and better understood using novel approaches.
I hypothesize that the interactions within MTBC and different human populations have imprinted the MTBC genome and thus, we aim to find the specific genomic drivers of tuberculosis in humans which could explains the restriction of M. africanum to west African populations.
This proposal can offer which processes are implicated in host specificity that might be responsible for virulence in specific hosts and constitutes a novel way using evolutionary theory to decipher aspects of tuberculosis disease.
Mireia Coscollá
European Society of Clinical Microbiology and Infectious Diseases