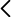Research group: TheSiMBioSys
Antibiotic resistance and tissue engineering/regenerative medicine have been acknowledged as two of the grand research challenges of Biotechnology. While seemingly unconnected, we argue that providing quantitative answers to these questions requires a common approach from the viewpoint of Mechanobiology. On the one hand, the characteristic phenotypic readout of antibiotic resistance (and more in general of a stress response in bacteria) is filamentation. The latter has been linked to survival strategies and virulence. Consequently, understanding the processes driving such distinctive phenotypic feature can open the door to develop solutions to tackle this problem and its consequences (e.g. biofilm formation). In this context, the role played by biomechanical cues during cell filamentation has been largely ignored. On the other hand, regenerative medicine depends on solutions from the field of tissue/organ engineering. The latter is a multifactorial problem that requires, among others, a deep understanding of the mechanisms shaping cell aggregates. Shaping a tissue in turn involves knowledge about how the cellular dynamics drives remodeling collectively. In that regard, cellular intercalation processes (either in time or in space) are key to understand tissue plasticity. However, as of today, no model has convincingly explained how stationary polarity patterns can be translated into a sustained planar intercalation dynamics (T1 transitions in time) and, in the context of our discovery of scutoids (T1 transitions in space), there is a lack of quantitative understanding about how forces are regulated and about the underlying molecular effectors. Specific aims of this project are, Aim 1: To understand the role played by mechanosignaling feedbacks during E. coli filamentation; Aim 2: To understand the coupling between tissue patterning/signaling in cellular intercalation events (in space and time). Specific open questions that we will address are a) the existence of mechanosensitive properties of the division/growth machinery in E. coli to regulate its filamentation and phenotypic diversity, b) the mechanisms that translate patterning properties into the elongation of the tissue during limb bud outgrowth, and c) the required methodology to characterize forces and energetic contributions in a three-dimensional (3D) tissue context, and the subsequent link to underlying effectors and the development of realistic tissue simulation schemes.
Javier Buceta
Ministerio de Ciencia e Innovacion