GEOGRAPHIC AND
VERTICAL DISTRIBUTION
Sedimentary vs. water-column materials
As opposed to other zooplanktonic
groups, studies on the geographic distribution of extant
polycystines have been chiefly based on sedimentary - rather than
on planktonic - materials. As mentioned above, sediment samples
present some advantages, but also several important shortcomings.
Whereas polycystine abundances
seldom exceed five cells per liter in the plankton (e.g., Caron and Swanberg 1990), one gram of (dry) surface sediments can
contain thousands to hundreds of thousands of radiolarian
skeletons. Plankton samples yield a snapshot-type image of the
composition of the assemblages, which does not necessarily
adequately reflect long-term trends. The daily, seasonal and
interannual variability involved is smoothed out in the
sedimentary record, which may be a welcome trait when general
patterns are sought. Further, sedimentary materials are more
readily available from the various repositories around the globe
than plankton samples. In any case, plankton samples not
collected for microplanktonic purposes may be useless for
radiolarian studies due to inadequate net mesh-size.
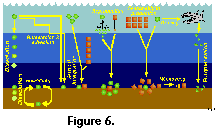 On the other hand, interpretation of the
geographic distribution of extant radiolarian assemblages on the
basis of sediment samples presents several important drawbacks (Boltovskoy 1988, 1994, 1995; Kling and Boltovskoy 1995; Figure 6). On their way to the sea-floor and after
settling, radiolarian remains are grazed upon by various
consumers thus breaking their skeletons into unidentifiable
fragments. Because more delicate shells are destroyed more
readily than the more robust ones, specific makeups on the bottom
and at mid- depths can differ significantly from the living
assemblage in the upper water-column (Boltovskoy et al. 1993b, 1995). Selective dissolution of whole siliceous
skeletons en route to the sea-floor and after deposition,
although often advocated as an important source of plankton vs.
sediments dissimilarities (e.g., Petrushevskaya 1971b; Renz 1976), is probably much less critical than
fragmentation due to grazing (Boltovskoy and Alder 1992; Morley et al. Ms). Bottom materials can be reworked after
deposition (as a result of which non-Recent deposits, sometimes
characteristic of quite dissimilar oceanographic settings, are
brought up to the surface layer, or winnowed by bottom currents
(dislodging settled skeletons and carrying them thousands of
kilometers away; Figure 6). Sediments integrate the imprint of
near-surface faunas (which are generally associated with
surficial temperature, salinity and primary production fields, as
well as with currents and water masses), with the meso- and
bathypelagic species whose geographic distribution is uncoupled
with upper-water oceanography (Figure 6). In general terms the
sedimentary distributions of cold-water species tend to show
conspicuous equatorward extensions as compared with their
planktonic patterns. This distortion is most probably due to the
fact that extended survival of the expatriated cold water taxa is
facilitated by submersion (Boltovskoy 1988, 1994;
Figure 6); as a consequence, sediment-derived species-specific
ranges may wrongly suggest an enhanced tolerance to gradients in
the ecological factors.
On the other hand, interpretation of the
geographic distribution of extant radiolarian assemblages on the
basis of sediment samples presents several important drawbacks (Boltovskoy 1988, 1994, 1995; Kling and Boltovskoy 1995; Figure 6). On their way to the sea-floor and after
settling, radiolarian remains are grazed upon by various
consumers thus breaking their skeletons into unidentifiable
fragments. Because more delicate shells are destroyed more
readily than the more robust ones, specific makeups on the bottom
and at mid- depths can differ significantly from the living
assemblage in the upper water-column (Boltovskoy et al. 1993b, 1995). Selective dissolution of whole siliceous
skeletons en route to the sea-floor and after deposition,
although often advocated as an important source of plankton vs.
sediments dissimilarities (e.g., Petrushevskaya 1971b; Renz 1976), is probably much less critical than
fragmentation due to grazing (Boltovskoy and Alder 1992; Morley et al. Ms). Bottom materials can be reworked after
deposition (as a result of which non-Recent deposits, sometimes
characteristic of quite dissimilar oceanographic settings, are
brought up to the surface layer, or winnowed by bottom currents
(dislodging settled skeletons and carrying them thousands of
kilometers away; Figure 6). Sediments integrate the imprint of
near-surface faunas (which are generally associated with
surficial temperature, salinity and primary production fields, as
well as with currents and water masses), with the meso- and
bathypelagic species whose geographic distribution is uncoupled
with upper-water oceanography (Figure 6). In general terms the
sedimentary distributions of cold-water species tend to show
conspicuous equatorward extensions as compared with their
planktonic patterns. This distortion is most probably due to the
fact that extended survival of the expatriated cold water taxa is
facilitated by submersion (Boltovskoy 1988, 1994;
Figure 6); as a consequence, sediment-derived species-specific
ranges may wrongly suggest an enhanced tolerance to gradients in
the ecological factors.
While these limitations stress the
need for caution in biogeographic interpretations based on
sedimentary materials, the usefulness of bottom samples for
biogeographic, paleobiogeographic and paleoecologic purposes has
been confirmed in many reports and is certainly beyond doubt.
Furthermore, when compared with water-column materials,
sedimentary ones can furnish much useful distributional and
ecological data (e.g., Boltovskoy et al. 1993b; 1995), usually unavailable from plankton or
sediment trap samples alone.
Geographic patterns
Polycystines are typically
open-ocean organisms, occurring throughout the World Ocean.
However, distinct coastal associations, while uncommon or absent
altogether in areas with an extended shelf, such as the
Southwestern Atlantic (Boltovskoy 1980), have been described in various studies.
For example, Norwegian fjords host dense and diverse radiolarian
assemblages, which differ from those of the open Norwegian Sea (Swanberg and Bjørklund
1986, 1987, 1992). Interestingly, two of these fjord
species, Rhizoplegma boreale (probably synonymous with Spongosphaera
streptacantha), and Phormacantha hystrix/Plectacantha
oikiskos, have been found to strongly dominate (up to 47% of
all polycystines) shallow, coastal sediments around Antarctica (Nishimura et al. 1997). General differences between presumably
neritic vs. oceanic radiolarian assemblages have been described
occasionally in the literature (Kruglikova 1984), and even used for paleoenvironmental
reconstructions (e.g., Palmer 1986). However, with the probable exception of
specific diversities, which indeed seem lower in neritic
assemblages (Nishimura et al. 1997), and the fact that a few selected
polycystines are probably less intolerant to near-shore
conditions than the bulk, most other traits (such as
Spumellaria:Nassellaria proportions, percentages of
Spongodiscidae, percentages of "spiny Porodiscidae",
percentages of small "Cyrtoidea"; cf. Kruglikova 1984) need further confirmation.
 Polycystine densities are typically around
0.3-1 cells per liter, but values exceeding 50 ind. l-1
have been recorded in some productive areas (Caron and Swanberg 1990). The quantitative distribution of
polycystines in surface sediments of the South Atlantic is
illustrated in Figure 7. This pattern is probably an approximate
representation of their concentrations in the water-column as
well, and it also roughly reflects the overall distribution of
primary production (e.g., Koblentz-Mishke and
Vedernikov 1977), and of
phytoplanktonic (Semina 1977) and zooplanktonic (Bogorov et al. 1968) biomasses. Highest numbers of polycystines
would thus be expected along the upwelling areas off Africa (Abelmann and Gowing 1997), where the highest radiolarian fluxes have
been recorded to day (Boltovskoy et al. 1996), and in the equatorial current system. In
the southern part of the ocean high densities are probably
associated with the subantarctic belt and its northern
extensions, the Malvinas (=Falkland) and the Benguela Currents.
In a transect between the Antarctic and approximately 30°S,
10°E (off Namibia), Abelmann and Gowing
(1997) recorded highest
polycystine densities at 100-300 m in Antarctic waters, and at
0-150 m in subantarctic waters (up to 0.3 ind. l-1;
these values, however, may be somewhat underestimating, see Boltovskoy and Alder 1992) . In the Southwestern Atlantic (30-60°S,
along 55°W), surface (5-15 m) layers were found to host 0.5
polycystines per liter on the average, with maximum
concentrations of three shells per liter (Alder et al. 1997). Lowest numbers are those present in
Central Gyre and Tropical/Subtropical waters (see Figure 7).
Polycystine densities are typically around
0.3-1 cells per liter, but values exceeding 50 ind. l-1
have been recorded in some productive areas (Caron and Swanberg 1990). The quantitative distribution of
polycystines in surface sediments of the South Atlantic is
illustrated in Figure 7. This pattern is probably an approximate
representation of their concentrations in the water-column as
well, and it also roughly reflects the overall distribution of
primary production (e.g., Koblentz-Mishke and
Vedernikov 1977), and of
phytoplanktonic (Semina 1977) and zooplanktonic (Bogorov et al. 1968) biomasses. Highest numbers of polycystines
would thus be expected along the upwelling areas off Africa (Abelmann and Gowing 1997), where the highest radiolarian fluxes have
been recorded to day (Boltovskoy et al. 1996), and in the equatorial current system. In
the southern part of the ocean high densities are probably
associated with the subantarctic belt and its northern
extensions, the Malvinas (=Falkland) and the Benguela Currents.
In a transect between the Antarctic and approximately 30°S,
10°E (off Namibia), Abelmann and Gowing
(1997) recorded highest
polycystine densities at 100-300 m in Antarctic waters, and at
0-150 m in subantarctic waters (up to 0.3 ind. l-1;
these values, however, may be somewhat underestimating, see Boltovskoy and Alder 1992) . In the Southwestern Atlantic (30-60°S,
along 55°W), surface (5-15 m) layers were found to host 0.5
polycystines per liter on the average, with maximum
concentrations of three shells per liter (Alder et al. 1997). Lowest numbers are those present in
Central Gyre and Tropical/Subtropical waters (see Figure 7).
Flux rates of radiolarian shells at
depths between 50 and ca. 5000 m vary from 0-4 to over 100,000
ind./m2/day (Boltovskoy et al. 1993a), with highest numbers having so far been
recorded in the north-eastern tropical Atlantic (201,064 shells/m2/day;
cf. Boltovskoy et al. 1996).
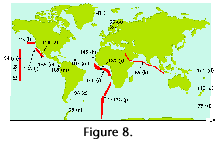 The numbers of species that inhabit the
different climatic zones of the World Ocean are difficult to
estimate because most authors restrict their scopes to some 20-40
more or less well-defined morphotypes, ignoring the rest of the
species. The few surveys that (presumably) did attempt to
identify all the skeletons recorded indicate that these numbers
oscillate around 100-200 for the tropics and subtropics, dropping
to some 50-60 at the poles (Figure 8).
The numbers of species that inhabit the
different climatic zones of the World Ocean are difficult to
estimate because most authors restrict their scopes to some 20-40
more or less well-defined morphotypes, ignoring the rest of the
species. The few surveys that (presumably) did attempt to
identify all the skeletons recorded indicate that these numbers
oscillate around 100-200 for the tropics and subtropics, dropping
to some 50-60 at the poles (Figure 8). 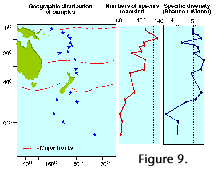 This decrease, however, is often punctuated
by an isolated peak in the transitional areas which usually host
both cold water and warm water taxa, especially in the sediments
(see Figure 9) (Boltovskoy 1981d, 1982, 1986).
This decrease, however, is often punctuated
by an isolated peak in the transitional areas which usually host
both cold water and warm water taxa, especially in the sediments
(see Figure 9) (Boltovskoy 1981d, 1982, 1986).
Despite these rather high numbers,
very few of the species are abundant in any given sample. In
terms of their relative contribution to the overall polycystine
assemblage, usually only one-three species exceed 10%, and up to
five represent over 5%; radiolarians whose average percentage
abundances are below 1% of the fauna usually comprise 70-90% of
all the species recorded (Figure 10). 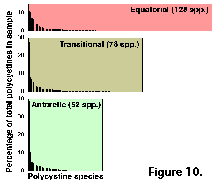 Of the 164 polycystines included in this
review, around 10 can attain average proportions in excess of 10%
in any given area, 12-15 morphotypes can reach 5-7%, and ca.
50-70 are normally around 1-3% (Table 1). The remaining half of the polycystine
species are present at levels below 1%. Highest dominances are
associated with polar environments, where a single species or
species group can account for 25-40% of the assemblage (e.g., Antarctissa
spp. in the Antarctic, cf. Boltovskoy 1987; Amphimelissa setosa in the
Greenland Sea, cf. Swanberg and Eide 1992; Phormacantha hystrix/Plectacantha
oikiskos and Rhizoplegma boreale, probably synoymous
with Spongosphaera streptacantha, in coastal Antarctic
sediments, cf. Nishimura et al. 1997; see Figure 10).
Of the 164 polycystines included in this
review, around 10 can attain average proportions in excess of 10%
in any given area, 12-15 morphotypes can reach 5-7%, and ca.
50-70 are normally around 1-3% (Table 1). The remaining half of the polycystine
species are present at levels below 1%. Highest dominances are
associated with polar environments, where a single species or
species group can account for 25-40% of the assemblage (e.g., Antarctissa
spp. in the Antarctic, cf. Boltovskoy 1987; Amphimelissa setosa in the
Greenland Sea, cf. Swanberg and Eide 1992; Phormacantha hystrix/Plectacantha
oikiskos and Rhizoplegma boreale, probably synoymous
with Spongosphaera streptacantha, in coastal Antarctic
sediments, cf. Nishimura et al. 1997; see Figure 10).
Species-specific distributional
data for the South Atlantic are scarce and fragmentary. Boltovskoy (1981e) produced a detailed listing of all known
Southwestern Atlantic records up to that date, which basically
represented 7 reports (Haeckel 1887; Hays 1965; Nigrini 1967; Goll and Bjørklund 1974; Lozano and Hays 1976; Morley 1977; and Boltovskoy and Riedel
1980), chiefly based on
sedimentary materials. This objective compilation produced a
spotty picture with no discernible patterns. In the 15 years
elapsed since that review several contributions based on South
Atlantic materials appeared, but they mostly focused on downcore
analyses (e.g., Pisias and Moore 1978; Coco 1982; Weaver 1983; Bjørklund and Jansen
1984; Grinstead 1984; Charles and Morley 1988; Alperín 1987), or were restricted geographically to
rather small areas (Robson 1983; Dworetzky and Morley 1987; Boltovskoy et al. 1993a, 1993b, 1995, 1996; Abelmann and Gowing 1997). 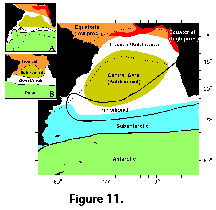 Thus, in order to furnish a more
comprehensive insight into polycystine biogeography in the South
Atlantic, distributional species-specific data are referred to
the 7 distinct areas illustrated in Figure 11). These divisions take into account the
distribution of general planktonic biogeographic provinces (e.g.,
E. Boltovskoy 1970; Koblentz-Mishke and
Vedernikov 1977; Boltovskoy 1979, 1981d,
1982, 1986; Dadon and Boltovskoy 1982; Longhurst 1995), as well as radiolarian-based
biogeographic patterns (Goll and Bjørklund 1974; Morley 1977; see Figure 11, insets A and B). For some
of the especially abundant and better defined taxa relative
(percentage) contributions to all polycystines can be predicted
with reasonable accuracy. For most others, however, only a very
rough indication of their numbers (abundant, present) can be
offered for the time being.
Thus, in order to furnish a more
comprehensive insight into polycystine biogeography in the South
Atlantic, distributional species-specific data are referred to
the 7 distinct areas illustrated in Figure 11). These divisions take into account the
distribution of general planktonic biogeographic provinces (e.g.,
E. Boltovskoy 1970; Koblentz-Mishke and
Vedernikov 1977; Boltovskoy 1979, 1981d,
1982, 1986; Dadon and Boltovskoy 1982; Longhurst 1995), as well as radiolarian-based
biogeographic patterns (Goll and Bjørklund 1974; Morley 1977; see Figure 11, insets A and B). For some
of the especially abundant and better defined taxa relative
(percentage) contributions to all polycystines can be predicted
with reasonable accuracy. For most others, however, only a very
rough indication of their numbers (abundant, present) can be
offered for the time being.
The information used to compile
Table 1 was not restricted to data from the South Atlantic Ocean,
but was extracted from many reports on various oceanic areas,
putting special emphasis on water column-based surveys (see
"Sedimentary vs. water-column materials" above).
Although very subtle differences between oceanic basins probably
do exist (Nigrini 1967; Goll and Bjørklund 1974), polycystine species are chiefly
restricted in their distribution by climatic and productivity
fields, rather than by ocean basins, as are most other pelagic
planktonic organisms. Thus, with very few exceptions, similar
assemblages characterize the equatorial circumglobal belt, the
subtropical zones of the two hemispheres, and the polar waters (Petrushevskaya 1971a). Geographic endemics are rare, probably
accounting for less than 5% of all the species (one outstanding
example is Antarctissa spp., Figure 15.104, which is
absent in the Arctic, but dominates both the plankton and the
sediments of the Antarctic zone).
It should be born in mind that the
degree of mixture between most of the areas shown in Figure 11 is
extremely large. For example, in the western South Atlantic the
Transition Zone stretches up to almost 15 degrees in latitude
(ca. 34-35°S to 47-48°S; Subantarctic species are regularly
found here in the same tows as the Subtropical representatives (E. Boltovskoy 1970, 1981a,
1981b;
E. Boltovskoy et al. 1996). Because the Brazil current is a southwest
flowing branch of the South Equatorial Current, tropical
assemblages differ little from the subtropical ones. Central Gyre
fauna is also very similar to the Tropical and Subtropical one,
yet these oligotrophic waters, characterized by very low overall
plankton abundances, host enhanced proportions of several
colonial radiolarians.
Vertical profiles
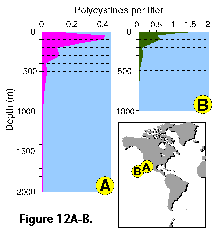 Vertical profiles of total radiolarian
abundance in tropical and subtropical waters indicate that the
bulk of their populations is usually located in the upper 50-100
m (Petrushevskaya 1971b; Renz 1976; Dworetzky and Morley 1987; Kling 1979; Kling and Boltovskoy 1995; Abelmann and Gowing 1997; see Figure 12A-B, 12C, 12F-G). Quite often several discrete maxima are
recorded, one at or near the surface, and a second one between 50
and 100 m (Petrushevskaya 1971b; Kling and Boltovskoy 1995).
Vertical profiles of total radiolarian
abundance in tropical and subtropical waters indicate that the
bulk of their populations is usually located in the upper 50-100
m (Petrushevskaya 1971b; Renz 1976; Dworetzky and Morley 1987; Kling 1979; Kling and Boltovskoy 1995; Abelmann and Gowing 1997; see Figure 12A-B, 12C, 12F-G). Quite often several discrete maxima are
recorded, one at or near the surface, and a second one between 50
and 100 m (Petrushevskaya 1971b; Kling and Boltovskoy 1995).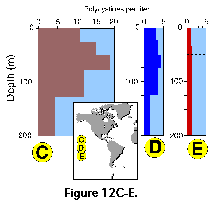 In the Antarctic, however, peak abundances
seem to be associated with the Warm Deep Water and occur deeper,
at 200-400 m (Petrushevskaya 1967; Boltovskoy and Alder 1992; Abelmann and Gowing 1997; Figure 12D, 12E).
In the Antarctic, however, peak abundances
seem to be associated with the Warm Deep Water and occur deeper,
at 200-400 m (Petrushevskaya 1967; Boltovskoy and Alder 1992; Abelmann and Gowing 1997; Figure 12D, 12E).
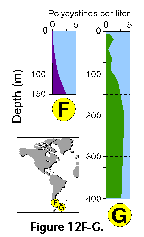 Many radiolarian species occupy discrete
depth intervals of the water column. Kling and Boltovskoy
(1995), on the basis of a
series of plankton tows in the upper 2000 m in the eastern
subtropical Pacific defined the following characteristic layers:
(1) surface (with maxima at 0 m, 25 m, 0 and 50 m, 50 m, or 0 and
100 m), (2) subsurface (maximum at 100 m), (3) deep (maxima at
200 m, 200 and 300 m, or 300 m), and (4) species peaking below
300 m. Roughly similar zonations were established by other
authors as well (e.g., Renz 1976; Dworetzky and Morley 1987; Kling 1979). Worldwide depth zonations, however,
cannot be defined in terms of fixed depths because the
distribution of radiolarian species is related to water masses
which move vertically as well as horizontally. For example, in
the eastern subtropical Pacific inshore and oceanic 0-25 m waters
can host a typically warm-water assemblage associated with the
Central Water which is advected coastward by the Southern
California Eddy, while midway between these two sites the same
depths are inhabited by a conspicuously different, colder-water
assemblage associated with the cooler waters of the California
Current (Kling and Boltovskoy 1995). Many cold water radiolarians that inhabit
the upper layers at high latitudes submerge with their
corresponding water masses and can be found at depth in mid- and
low-latitude areas (Kling 1976; Boltovskoy 1988; Steineck and Casey 1990). Siphocampe arachnea (Figure
15.167), for example, is a dominant component of surface Pacific
Arctic and Subarctic plankton; in the central north Pacific it
peaks at 100-300 m, and at 300-1000 m in the subtropical eastern
Pacific (Boltovskoy 1994).
Many radiolarian species occupy discrete
depth intervals of the water column. Kling and Boltovskoy
(1995), on the basis of a
series of plankton tows in the upper 2000 m in the eastern
subtropical Pacific defined the following characteristic layers:
(1) surface (with maxima at 0 m, 25 m, 0 and 50 m, 50 m, or 0 and
100 m), (2) subsurface (maximum at 100 m), (3) deep (maxima at
200 m, 200 and 300 m, or 300 m), and (4) species peaking below
300 m. Roughly similar zonations were established by other
authors as well (e.g., Renz 1976; Dworetzky and Morley 1987; Kling 1979). Worldwide depth zonations, however,
cannot be defined in terms of fixed depths because the
distribution of radiolarian species is related to water masses
which move vertically as well as horizontally. For example, in
the eastern subtropical Pacific inshore and oceanic 0-25 m waters
can host a typically warm-water assemblage associated with the
Central Water which is advected coastward by the Southern
California Eddy, while midway between these two sites the same
depths are inhabited by a conspicuously different, colder-water
assemblage associated with the cooler waters of the California
Current (Kling and Boltovskoy 1995). Many cold water radiolarians that inhabit
the upper layers at high latitudes submerge with their
corresponding water masses and can be found at depth in mid- and
low-latitude areas (Kling 1976; Boltovskoy 1988; Steineck and Casey 1990). Siphocampe arachnea (Figure
15.167), for example, is a dominant component of surface Pacific
Arctic and Subarctic plankton; in the central north Pacific it
peaks at 100-300 m, and at 300-1000 m in the subtropical eastern
Pacific (Boltovskoy 1994).
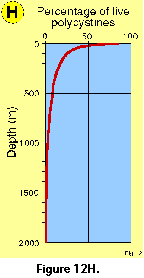 Changes in the proportions of presumably
living polycystine cells with depth have been assessed in a few
studies. Boltovskoy et al. (1993a), based on extensive sediment trap data,
concluded that numbers of live specimens decrease drastically
downwards (e.g., aprox. 100% at 0 m, 50-60% at 100 m, 20-40% at
200 m, 10-20% at 500 m, 5% at 1000 m; see Figure
12H). These results
generally agreee with other studies (e.g., Petrushevskaya 1971a; Kling and Boltovskoy 1995). On the other hand, Abelmann and Gowing
(1997) estimated much
higher proportions of living cells at comparable levels in the
water column: over 90% at 100-200 m, around 70% at 300-500 m. It
should be noticed that staining techniques, which are usually
applied for these estimates, do not adequately differentiate
between live and dead cells (see above "Provenance and
collection of materials"), for which reason it is probable
that concentrations of living specimens below 50-100 m are
systematically overestimated in such surveys (Boltovskoy et al. 1993a; see Figure 12H).
Changes in the proportions of presumably
living polycystine cells with depth have been assessed in a few
studies. Boltovskoy et al. (1993a), based on extensive sediment trap data,
concluded that numbers of live specimens decrease drastically
downwards (e.g., aprox. 100% at 0 m, 50-60% at 100 m, 20-40% at
200 m, 10-20% at 500 m, 5% at 1000 m; see Figure
12H). These results
generally agreee with other studies (e.g., Petrushevskaya 1971a; Kling and Boltovskoy 1995). On the other hand, Abelmann and Gowing
(1997) estimated much
higher proportions of living cells at comparable levels in the
water column: over 90% at 100-200 m, around 70% at 300-500 m. It
should be noticed that staining techniques, which are usually
applied for these estimates, do not adequately differentiate
between live and dead cells (see above "Provenance and
collection of materials"), for which reason it is probable
that concentrations of living specimens below 50-100 m are
systematically overestimated in such surveys (Boltovskoy et al. 1993a; see Figure 12H).
As with geographic patterns, data
on the depths at which the various species peak listed in Table 1
have been compiled from reports on different oceanic areas. It is
anticipated that they are generally valid for subtropical and
tropical environments worldwide; at higher latitudes, however,
some deep species may occur closer to the surface, while in the
Antarctic the bulk of the asssemblages seems to occupy deeper
layers (see above).
 On the other hand, interpretation of the
geographic distribution of extant radiolarian assemblages on the
basis of sediment samples presents several important drawbacks (Boltovskoy 1988, 1994, 1995; Kling and Boltovskoy 1995; Figure 6). On their way to the sea-floor and after
settling, radiolarian remains are grazed upon by various
consumers thus breaking their skeletons into unidentifiable
fragments. Because more delicate shells are destroyed more
readily than the more robust ones, specific makeups on the bottom
and at mid- depths can differ significantly from the living
assemblage in the upper water-column (Boltovskoy et al. 1993b, 1995). Selective dissolution of whole siliceous
skeletons en route to the sea-floor and after deposition,
although often advocated as an important source of plankton vs.
sediments dissimilarities (e.g., Petrushevskaya 1971b; Renz 1976), is probably much less critical than
fragmentation due to grazing (Boltovskoy and Alder 1992; Morley et al. Ms). Bottom materials can be reworked after
deposition (as a result of which non-Recent deposits, sometimes
characteristic of quite dissimilar oceanographic settings, are
brought up to the surface layer, or winnowed by bottom currents
(dislodging settled skeletons and carrying them thousands of
kilometers away; Figure 6). Sediments integrate the imprint of
near-surface faunas (which are generally associated with
surficial temperature, salinity and primary production fields, as
well as with currents and water masses), with the meso- and
bathypelagic species whose geographic distribution is uncoupled
with upper-water oceanography (Figure 6). In general terms the
sedimentary distributions of cold-water species tend to show
conspicuous equatorward extensions as compared with their
planktonic patterns. This distortion is most probably due to the
fact that extended survival of the expatriated cold water taxa is
facilitated by submersion (Boltovskoy 1988, 1994;
Figure 6); as a consequence, sediment-derived species-specific
ranges may wrongly suggest an enhanced tolerance to gradients in
the ecological factors.
On the other hand, interpretation of the
geographic distribution of extant radiolarian assemblages on the
basis of sediment samples presents several important drawbacks (Boltovskoy 1988, 1994, 1995; Kling and Boltovskoy 1995; Figure 6). On their way to the sea-floor and after
settling, radiolarian remains are grazed upon by various
consumers thus breaking their skeletons into unidentifiable
fragments. Because more delicate shells are destroyed more
readily than the more robust ones, specific makeups on the bottom
and at mid- depths can differ significantly from the living
assemblage in the upper water-column (Boltovskoy et al. 1993b, 1995). Selective dissolution of whole siliceous
skeletons en route to the sea-floor and after deposition,
although often advocated as an important source of plankton vs.
sediments dissimilarities (e.g., Petrushevskaya 1971b; Renz 1976), is probably much less critical than
fragmentation due to grazing (Boltovskoy and Alder 1992; Morley et al. Ms). Bottom materials can be reworked after
deposition (as a result of which non-Recent deposits, sometimes
characteristic of quite dissimilar oceanographic settings, are
brought up to the surface layer, or winnowed by bottom currents
(dislodging settled skeletons and carrying them thousands of
kilometers away; Figure 6). Sediments integrate the imprint of
near-surface faunas (which are generally associated with
surficial temperature, salinity and primary production fields, as
well as with currents and water masses), with the meso- and
bathypelagic species whose geographic distribution is uncoupled
with upper-water oceanography (Figure 6). In general terms the
sedimentary distributions of cold-water species tend to show
conspicuous equatorward extensions as compared with their
planktonic patterns. This distortion is most probably due to the
fact that extended survival of the expatriated cold water taxa is
facilitated by submersion (Boltovskoy 1988, 1994;
Figure 6); as a consequence, sediment-derived species-specific
ranges may wrongly suggest an enhanced tolerance to gradients in
the ecological factors. Polycystine densities are typically around
0.3-1 cells per liter, but values exceeding 50 ind. l-1
have been recorded in some productive areas (
Polycystine densities are typically around
0.3-1 cells per liter, but values exceeding 50 ind. l-1
have been recorded in some productive areas ( The numbers of species that inhabit the
different climatic zones of the World Ocean are difficult to
estimate because most authors restrict their scopes to some 20-40
more or less well-defined morphotypes, ignoring the rest of the
species. The few surveys that (presumably) did attempt to
identify all the skeletons recorded indicate that these numbers
oscillate around 100-200 for the tropics and subtropics, dropping
to some 50-60 at the poles (
The numbers of species that inhabit the
different climatic zones of the World Ocean are difficult to
estimate because most authors restrict their scopes to some 20-40
more or less well-defined morphotypes, ignoring the rest of the
species. The few surveys that (presumably) did attempt to
identify all the skeletons recorded indicate that these numbers
oscillate around 100-200 for the tropics and subtropics, dropping
to some 50-60 at the poles ( This decrease, however, is often punctuated
by an isolated peak in the transitional areas which usually host
both cold water and warm water taxa, especially in the sediments
(see
This decrease, however, is often punctuated
by an isolated peak in the transitional areas which usually host
both cold water and warm water taxa, especially in the sediments
(see  Of the 164 polycystines included in this
review, around 10 can attain average proportions in excess of 10%
in any given area, 12-15 morphotypes can reach 5-7%, and ca.
50-70 are normally around 1-3% (
Of the 164 polycystines included in this
review, around 10 can attain average proportions in excess of 10%
in any given area, 12-15 morphotypes can reach 5-7%, and ca.
50-70 are normally around 1-3% ( Thus, in order to furnish a more
comprehensive insight into polycystine biogeography in the South
Atlantic, distributional species-specific data are referred to
the 7 distinct areas illustrated in
Thus, in order to furnish a more
comprehensive insight into polycystine biogeography in the South
Atlantic, distributional species-specific data are referred to
the 7 distinct areas illustrated in  Vertical profiles of total radiolarian
abundance in tropical and subtropical waters indicate that the
bulk of their populations is usually located in the upper 50-100
m (
Vertical profiles of total radiolarian
abundance in tropical and subtropical waters indicate that the
bulk of their populations is usually located in the upper 50-100
m ( In the Antarctic, however, peak abundances
seem to be associated with the Warm Deep Water and occur deeper,
at 200-400 m (
In the Antarctic, however, peak abundances
seem to be associated with the Warm Deep Water and occur deeper,
at 200-400 m ( Many radiolarian species occupy discrete
depth intervals of the water column.
Many radiolarian species occupy discrete
depth intervals of the water column.  Changes in the proportions of presumably
living polycystine cells with depth have been assessed in a few
studies.
Changes in the proportions of presumably
living polycystine cells with depth have been assessed in a few
studies.