Eighty-two samples were analyzed from 13 bones in Giganotosaurus
compared to 54 samples from 12 bones in T. rex (Barrick
and Showers, 1994). The proximal and
distal ends of a rib, femur, tibia and pubis were sampled to search for
temperature trends along the length of these bones. Heterogeneity in the oxygen
isotope value of bone phosphate (![]()
![]() p)
within skeletal elements is used to calculate intrabone temperature variability
while differences in the mean values between skeletal elements are used to
determine interbone temperature differences. For these calculations,
p)
within skeletal elements is used to calculate intrabone temperature variability
while differences in the mean values between skeletal elements are used to
determine interbone temperature differences. For these calculations, ![]()
![]() p
was multiplied by the slope of Longinelli
and Nuti’s (1973) phosphate
paleotemperature equation (i.e., 4.3).
p
was multiplied by the slope of Longinelli
and Nuti’s (1973) phosphate
paleotemperature equation (i.e., 4.3).
An assessment of the isotopic integrity of the
fossil bone material was made using comparisons of the co-existing structural
carbonate (![]() sc),
secondary calcite cements (
sc),
secondary calcite cements (![]() cc)
with the bone phosphate (Fig. 1.1-3
cc)
with the bone phosphate (Fig. 1.1-3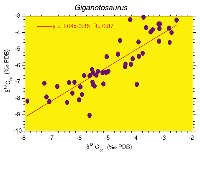 )
as suggested by (Barrick
and Showers 1995; Barrick
et al. 1996; Barrick
1998). The carbon isotope signature
indicates that after burial, during recrystallization of the bone apatite
crystals, the structural carbonate ions were exchanged with carbonate ions from
the diagenetic groundwater (r=0.87) and that oxygen atoms within the ions were
exchanged (r=0.80). The solubility of apatite decreases significantly after
recrystallization (Grupe
1988; Trueman
and Benton 1997) essentially making the
structural carbonate of the recrystallized carbonate fluorapatite impervious to
secondary diagenetic events as it also does to trace elements incorporated
during recrystallization (Williams
1988; Wright
et al. 1987; Trueman
and Benton 1997). The pronounced
covariation of cement to structural carbonate carbon and oxygen isotope values
precludes more than one major diagenetic event affecting the isotopic
composition of the structural carbonate and carbonate cements. If the phosphate
ions were significantly exchanged during this process, it is expected that
)
as suggested by (Barrick
and Showers 1995; Barrick
et al. 1996; Barrick
1998). The carbon isotope signature
indicates that after burial, during recrystallization of the bone apatite
crystals, the structural carbonate ions were exchanged with carbonate ions from
the diagenetic groundwater (r=0.87) and that oxygen atoms within the ions were
exchanged (r=0.80). The solubility of apatite decreases significantly after
recrystallization (Grupe
1988; Trueman
and Benton 1997) essentially making the
structural carbonate of the recrystallized carbonate fluorapatite impervious to
secondary diagenetic events as it also does to trace elements incorporated
during recrystallization (Williams
1988; Wright
et al. 1987; Trueman
and Benton 1997). The pronounced
covariation of cement to structural carbonate carbon and oxygen isotope values
precludes more than one major diagenetic event affecting the isotopic
composition of the structural carbonate and carbonate cements. If the phosphate
ions were significantly exchanged during this process, it is expected that ![]() p
will covary with
p
will covary with ![]() cc
or
cc
or ![]() sc
indicating re-equilibration with the
diagenetic fluids. This is not the case as seen in Figure
1.3 where the covariance is very weak (r=0.02), similar to the case seen in T.
rex. Thus, complete equilibration of
sc
indicating re-equilibration with the
diagenetic fluids. This is not the case as seen in Figure
1.3 where the covariance is very weak (r=0.02), similar to the case seen in T.
rex. Thus, complete equilibration of ![]() p
with diagenetic fluids is precluded. In
addition, an isotopic comparison was made between the cancellous and compact
bone samples. Cancellous bone is more susceptible to alteration than compact
bone because of its greater exposed surface area as shown in rare earth element
studies (Grupe 1988).
Thus, partial alteration of
p
with diagenetic fluids is precluded. In
addition, an isotopic comparison was made between the cancellous and compact
bone samples. Cancellous bone is more susceptible to alteration than compact
bone because of its greater exposed surface area as shown in rare earth element
studies (Grupe 1988).
Thus, partial alteration of ![]() p
would result in different isotope ratios
between the cancellous and compact bone. This is not apparent, for the mean
p
would result in different isotope ratios
between the cancellous and compact bone. This is not apparent, for the mean ![]() p
for 48 dense samples is 17.4‰ with a s1
of 0.66 and 34 cancellous samples have a mean value of 17.5‰ with a
p
for 48 dense samples is 17.4‰ with a s1
of 0.66 and 34 cancellous samples have a mean value of 17.5‰ with a ![]() 1
of 0.50. There is a 2.2‰ total range in the isotopic values of Giganotosaurus
while only a 0.1‰ difference in the mean dense and cancellous
1
of 0.50. There is a 2.2‰ total range in the isotopic values of Giganotosaurus
while only a 0.1‰ difference in the mean dense and cancellous ![]() 18Op
values. This precludes partial alteration of the
18Op
values. This precludes partial alteration of the ![]() 18Op
values and thus in conjunction with the co-existing carbonate and phosphate
data, isotopic preservation is interpreted for these bones.
18Op
values and thus in conjunction with the co-existing carbonate and phosphate
data, isotopic preservation is interpreted for these bones.