- "A two-scale approach to electron correlation in multiconfigurational perturbation theory".
P. Farahani, D. Roca-Sanjuán, and F. Aquilante,
Journal of Computational Chemistry, 35, 1609-1617 (2014)
On page 1609 (DOI: 10.1002/jcc.23666), Pooria Farahani et al. present a new approach for calculating dynamic electron correlation effects in large molecular systems using multiconfigurational second order perturbation theory (CASPT2). The method is restricted to cases where partitioning of the molecular system into an active site and an environment is meaningful. Only dynamic correlation effects derived from orbitals extending over the active site are included at the CASPT2 level of theory, whereas the correlation effects of the environment are retrieved at lower computational costs.

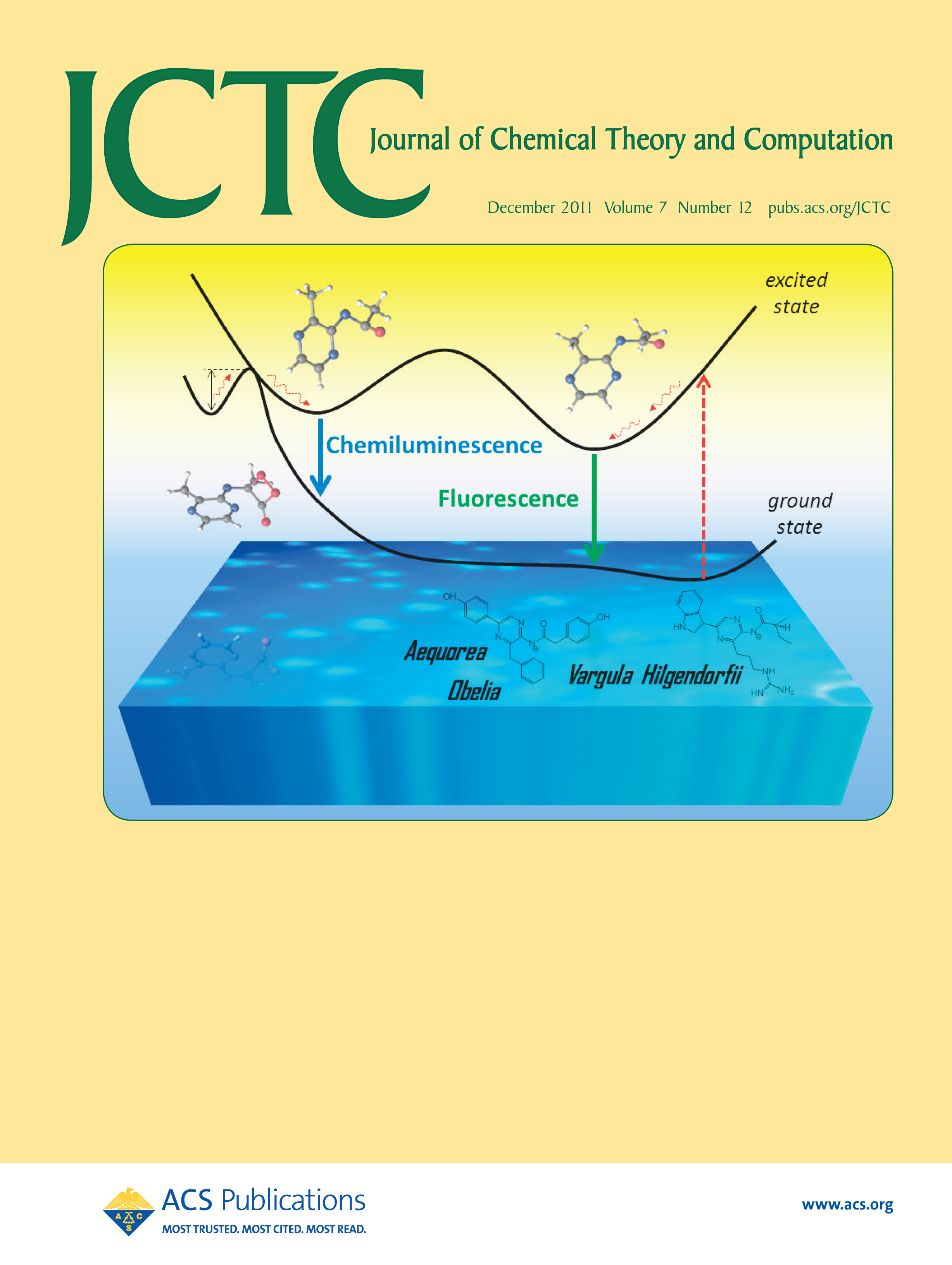
- "Chemiluminescence and Fluorescence of a Small Model for Coelenteramide and Cypridina Oxyluciferin: A CASSCF/CASPT2 Study".
D. Roca-Sanjuán, M. G. Delcey, I. Navizet, N. Ferré, Y.-J. Liu, and R. Lindh,
Journal of Chemical Theory and Computation, 7, 4060-4069 (2011)
Excited-state potential energy surfaces are often more complex than expected. The excited-state equilibrium minima responsible for the fluorescence and chemiluminescence phenomena in a small model for coelenteramide and Cypridina oxyluciferin have been found to be different. Such molecules are the light-emitting species in the Aequorea, Obelia, and Vargula hilgendorfii marine bioluminescent organisms. On the basis of the findings, some recommendations are given for future theoretical and experimental investigations on chemiluminescence and bioluminescence. See D. Roca-Sanjuán, M. G. Delcey, I. Navizet, N. Ferré, Y.-J. Liu, and R. Lindh, pp 4060—4069.
- "The Chemistry of Bioluminescence: An Analysis of Chemical Functionalities".
I. Navizet, Y.-J. Liu, N. Ferré, D. Roca-Sanjuán, and R. Lindh,
ChemPhysChem. A European Journal of Chemical Physics and Physical Chemistry, 12, 3064-3076 (2011)
The chemical functionalities of the bioluminescence phenomenon have been rationalized in the last decades via systematic studies from small to large-size molecular models, as reviewed by R. Lindh et al. on p. 3064. Electron-donating chemical groups conjugated to peroxo moieties are the molecular structures that intrinsically holds the optimal electronic properties for the process, whereas the surrounding enzymes mainly account for color-tuning.

- "MOLCAS 7: The Next Generation" .
F. Aquilante, L. De Vico, N. Ferré, G. Ghigo, P-.A. Malmqvist, T. Pedersen, M. Pitonak, M. Reiher, B. O. Roos, L. Serrano-Andrés, M. Urban, V. Veryazov, and R. Lindh,
Journal of Computational Chemistry, 31, 224-247 (2010)
Some of the new unique features of the MOLCAS quantum chemistry package version 7 are presented in this report. In particular, the Cholesky decomposition method applied to some quantum chemical methods is described. The report further elaborates on the implementation of a restrictive-active space self-consistent field reference function in conjunction with 2nd order perturbation theory. The average atomic natural orbital basis for relativistic calculations, covering the whole periodic table, are describe and associated unique properties are demonstrated. Furthermore, the use of the arbitrary order Douglas-Kroll-Hess transformation for one-component relativistic calculations and its implementation is discussed. Moreover, the ElectroStatic Potential Fitted scheme, a version of a quantum mechanics/molecular mechanics hybrid method implemented in MOLCAS, is described and discussed. Finally, the report discusses the use of the MOLCAS package for advanced studies of photo chemical phenomenon and the usefulness of the algorithms for constrained geometry optimization in MOLCAS in association with such studies.

- "Deciphering Intrinsic Deactivation/Isomerization Routes in a Phytochrome Chromophore Model".
P. Altoé, T. Climent, G. De Fusco, M. Stenta, A. Botoni, L. Serrano-Andrés, M. Merchán, G. Orlandi, and M. Garavelli,
The Journal of Physical Chemistry B, 113, 15067-15073 (2009)
Theoretical investigation of the multichannel photoisomerization mechanisms in the phytochrome chromophore. Crystallographic structure of the chromophore binding domain of the bacteriophytochrome (top right) and cartoonlike representation of competitive photoreaction paths in phytochrome chromophores according to the CASPT2//CIS scenario.
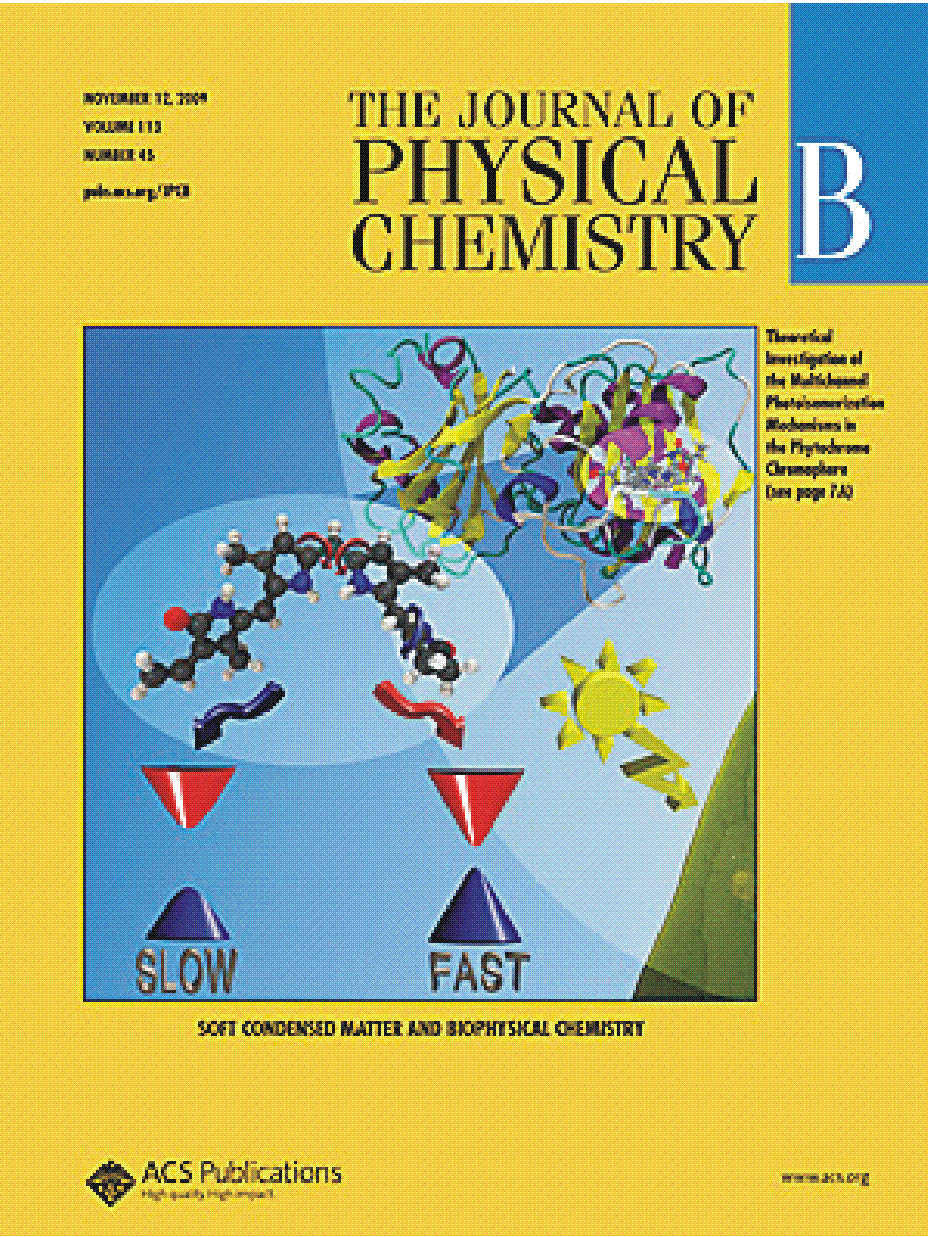
- "Do Fluorescence and Transient Absorption Probe the Same Intramolecular Charge Transfer State of 4-(Dimethylamino)benzonitrile?".
T. Gustavsson, P. B. Coto, L. Serrano-Andrés, T. Fujiwara, and E. C. Lim,
Journal of Chemical Physics, 131, 031101 (2009)
We present here the results of time-resolved absorption and emission experiments for 4-dimethylaminobenzonitrile in solution, which suggest that the fluorescent intramolecular charge transfer (ICT) state may differ from the twisted ICT (TICT) state observed in transient absorption.