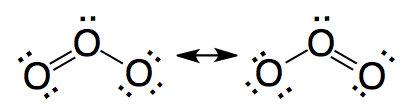

The valence bond model is unable to explain satisfactorily the electron distribution in molecules like molecule of O3. The resonant structures drawn do not represent real structures in equilibrium. The structure of O3 is an intermediate structure between the resonant structures that has not a simple representation with the electron dots representation.
The molecular orbital model describes the resonance locating this electron pair in a molecular orbital delocalized to the three atoms.