Equilibrium 15
The figure shows a system of two species in equilibrium: 2 NO2(g)⇄ N2O4(g) with a value of ΔH=-57 kJ/mol.
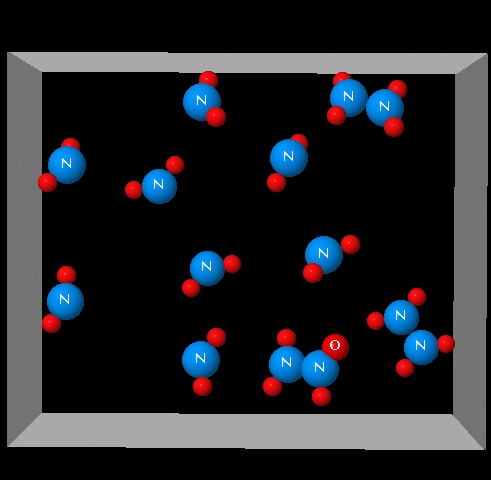 |
Click on the figure and observe the visualization shown. Indicate which of the following variables it may have caused the observed changes in the system?
The container is flexible and adapts to the system pressure as a rubber balloon does.
| Decreased external pressure | Wrong. In this process
the external pressure remains constant
|
| Decreased temperature | Right. The
formation of N2O4(g) is accompanied by a heat release. A decrease
in temperature results in the formation of N2O4(g) decreasing the
number of gaseous molecules.
|
| Increased temperature. | Wrong. This would produce
an increase of gas molecules and therefore an increase of pressure.
|