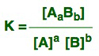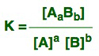A reaction is said to be reversible if it goes in the forward direction,
A + B forming AB, and also in the reverse one, AB dissociates into A + B.
When the rate of both forward and reverse reactions is the same, the reaction reaches a dynamic equilibrium
and concentrations of all species remain constant.
This equilibrium can be reached starting from A and B, but also starting from AB. The
situation at the time of equilibrium is given by the constant K whose value only depends on the temperature.
For a reaction such as: aA + bB ⇄ AaBb the equilibrium constant will be: