Reaction 1a
The following figure shows a sample containing molecules of Cl2 (green) and H2 (white).
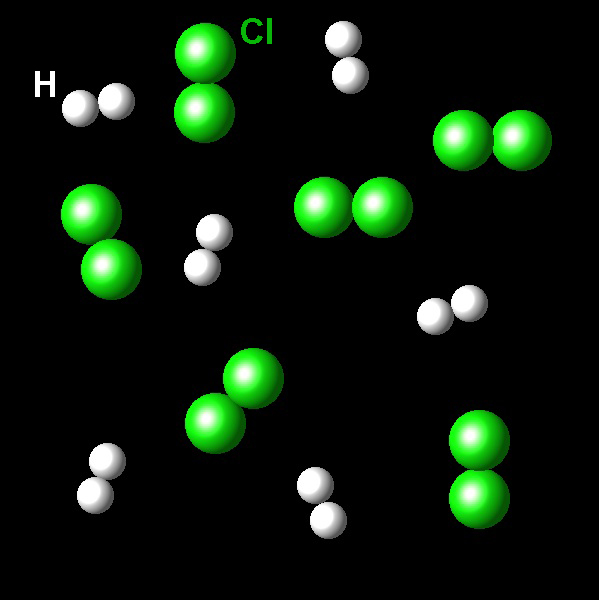 |
These molecules may react according to the following reaction: H2(g) + Cl2(g)→2HCl(g). Select the limiting reagent in this mixture.
Wrong. The stoichiometry of the reaction is 1:1. Check the number of molecules of each kind in the sample.
|
|
Wrong. The stoichiometry of the reaction is 1:1. Check the number of molecules of each kind in the sample.
|
|
Correct. The stoichiometry of the reaction is 1:1. The sample has the same number of molecules of hydrogen and chlorine. The mixture is already in the stoichiometric proportions
.
|