Reaction 2a
The following figure shows a fragment of a sample that contains oxygen molecules, O2, (red spheres) and hydrogen molecules, H2, (white spheres).
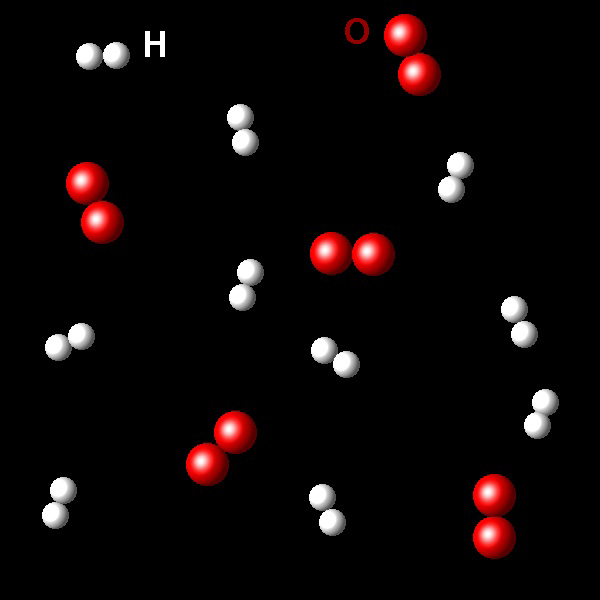 |
If this mixture rects to form water, select what is the limiting reagent in this conditions,
Right. The stoichiometry of this reaction
is two hydrogens molecules per one oxygen molecule. In this sample there are 10 molecules
of hydrogen and 6 molecules of oxygen. Hydrogen is the limiting reagent.
|
|
Wrong. The stoichiometry of the reaction is two
hydrogens molecules per one oxygen molecule. In this sample there are 10 molecules
of hydrogen and 6 molecules of oxygen. Hydrogen is the limiting reagent.
|
|
Wrong. The stoichiometric ratio
(H2/O2) is 10/5 = 2/1. There is a defect of hydrogen.
|