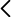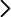A study published by four research groups from the UV, the University of La Laguna and California Los Angeles and the Institute of Chemical Technology reveals that carbon dioxide at high pressure and temperatures interact differently with metals such as gold, platinum and rhenium. The work, based on laboratory experiments, is relevant for the research of new carbon compounds which are part of the terrestrial mantle. Furthermore, this work also suggests a new methodology for these types of experiments.

The importance of this research is rooted in the great amount of carbon dioxide (CO2) accumulated in the earth crust in the form of carbonates in the areas of subduction (where oceanic plates join) which are connected to the terrestrial mantle. Due to the importance of this gas in the temperature of the Earth and as a result of its effect on climate change, to know the stability of the oxidised forms of carbon and the existence of this gas in the terrestrial mantle are questions of great relevance in geophysics (science which studies all the phenomena related to the structure, physic conditions and evolution of the Earth).
David Santamaría Pérez, researcher of the Material Sciences Institute and of the Department of Applied Physics and Electromagnetism of the UV, is the first signatory of the two articles about this research which have been published in the magazines Inorganic Chemistry and Nature Communications. These articles analyse the behaviour of carbon dioxide under extreme conditions of pressure and temperature on the terrestrial mantle, aimed at knowing in detail its chemistry.
“The first step to understand the behaviour of carbonate minerals, that is, compounds based on oxygen and carbon in the conditions of the inside of the Earth, is to reveal the chemical reactivity of CO2 in different environments. The laboratory experiments study in depth this knowledge and enable the design of new strategies to reproduce the conditions of the inner Earth, without having unwanted reaction products”, explained David Santamaría.
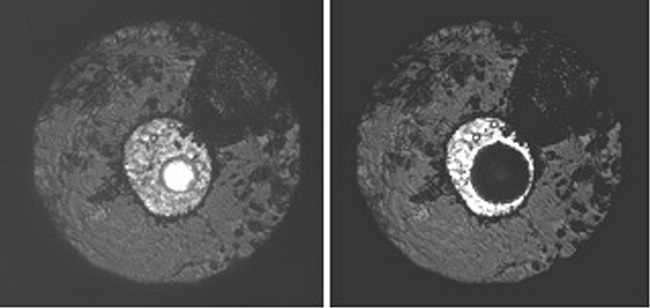
In order to study this behaviour, some laboratory experiments are carried out in pressure chambers which are made of metal compounds such as rhenium. The work developed by the group coordinated by David Santamaría found that this metal reacts with carbon dioxide at high pressure and that they form rhenium oxide. Contrarily, no reaction of carbon dioxide is found when compounds made of gold or platinum are used.
These results led to the rejection of the conclusions of a previous work published in the magazine Nature Communications on the formation of a new solid solution, a silicon and carbon oxide. “This work opened the door to a new chemistry at low pressures. The reported compound was potentially relevant for the fields of Earth and Material Sciences. However, our work shows that the compound which was formed at those pressures was in fact rhenium oxide, a result of the reaction with the pressure chamber of the experimental device”, claims David Santamaría.
The results of the published research make possible to suggest a new methodology to carry out experiments with CO2 and carbonate minerals at high pressure and temperature, avoiding the use of rhenium in pressure chambers. Furthermore, this methodology has been used to look for new compounds which could be potentially stable in experimental studies with which the behaviour at high temperatures and pressure of the system integrated by carbon dioxide and silicon dioxide is explored.
Photonics and Semiconductors
The Unit of Semiconductors under Extreme Conditions (Photonics and Semiconductors Group) of the University Institute of Materials Science (ICMUV) is specialised in the study of materials in different thermodynamic conditions. They have published numerous articles on optical and structural properties of technological materials (nanocrystalline oxides, hard or ferroelectric materials, among others).
Articles:
[1] D. Santamaria-Perez, C. McGuire, et al. (2016) Exploring the chemical reactivity between carbon dioxide and three transition metals (Au, Pt, and Re) at high pressures and temperatures. Inorg. Chem. 55, 10793.
http://pubs.acs.org/doi/abs/10.1021/acs.inorgchem.6b01858
[2] D. Santamaria-Perez, C. McGuire, et al. (2016) Strongly-driven Re + CO2 redox reaction at high-pressure, high-temperature conditions. Nature Communications 7:13647.
http://www.nature.com/articles/ncomms13647
Images: