Manuela Merchán
Luis Serrano-Andrés
Juan José Serrano-Pérez
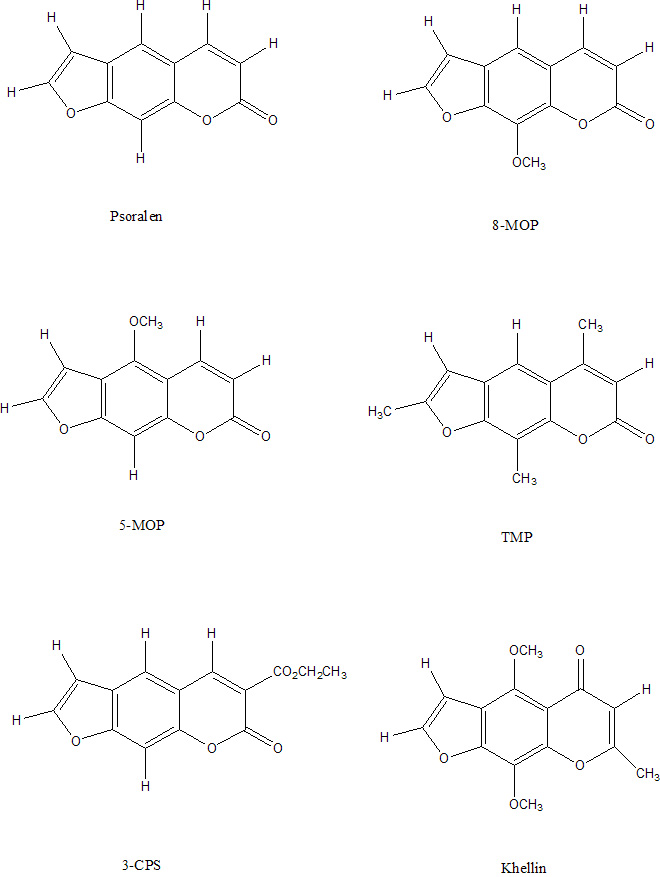
More recently, researchers have found useful applications for
psoralens as molecular probes in studies dealing with chromatin
structure, secondary structure in viral DNA and Drosophila
ribosomal RNA, satellite DNA structure, bacterial and viral DNA repair
mechanism, viral DNA-RNA hybrid structure and virus RNA duplexes.
The molecular basis for photobiological (skin photosensitization) and
photochemotherapeutic effects of psoralens involve the stable covalent
photoconjugation of furocoumarins with DNA of the target cells giving
rise to both the monofunctional (single strand) adducts and
interstrand cross-link adducts between the pyrimidine bases belonging
to opposite strands of DNA. The photochemotherapeutic effectiveness of
psoralen plus UVA is believe to be due: (a) the inhibition of DNA
synthesis and subsequent decrease in macromolecular synthesis, (b) the
killing of abnormal proliferative cells and the inhibition of
recruitment of new cells in the G0 and G2
phases, (c)the inhibition of blood vessel cells (angiogenic effect),
and (d) the inhibition of leukocytes and subsequent immunosuppression
effect on the immune system.
The major chemical reaction involving the photoconjugation of psoralen
and pyrimidine bases can be classified as a Type-I reaction that
requires the transfer of hydrogen atoms or electrons but no direct
involvement of molecular O2.
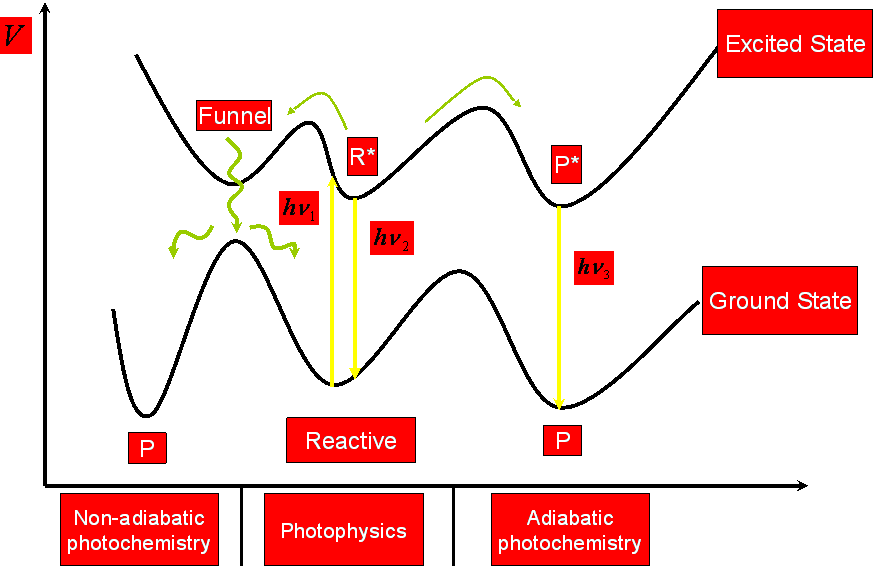
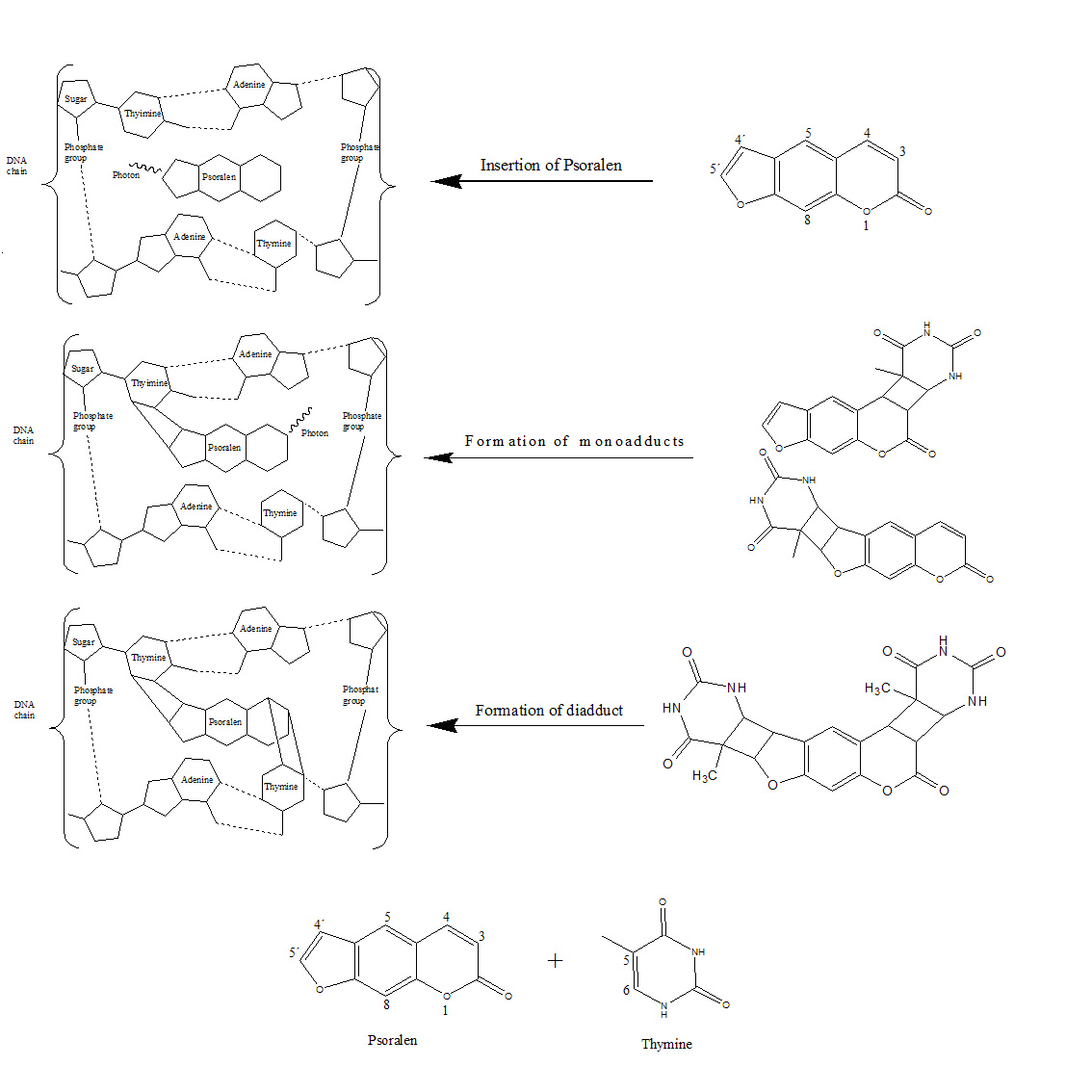
The photoreactive process is thought to involve three steps. The first step takes place in the dark: the psoralen insert themselves between adjacent thymine base pairs in the DNA duplex. In the second step, the psoralen compound absorbs a photon and forms a monoadduct. The psoralen compounds can form two types of monoadducts, the furan monoadduct and the pyrone monoadduct, by reacting with the C5-C6 double bond of the thymine via the C4´-C5´ double bond in the furan ring or the C3-C4 double bond in the pyrone ring. In the third step, the monoadduct also absorbs one photon, inducing the other photoreactive double bond of the monoadduct to react with a thymine on the opposite strand of DNA. Therefore, in the third step a diadduct that crosslinks the DNA is formed. The formation of the diadduct severely damages the DNA, requiring a long time for its restoration. The diadduct causes adverse effects and it is carcinogen and mugaten. Several studies have suggested that, whereas the furan monoadduct forms a diadduct, the pyrone monoadduct does not.
Psoralens also undergo Type-II reactions involving the formation of 1O2 and O2·- (or HO2·-) through a sensitized mechanism (photodynamic action) in which the photoexcited psoralen (in its triplet state) reacts with molecular oxygen to form reactive 1O2 or O2·-. These reactive forms of oxygen cause cell damage that eventually contributes to skin photosensitization, mutation, error-prone repair and skin carcinogenesis.
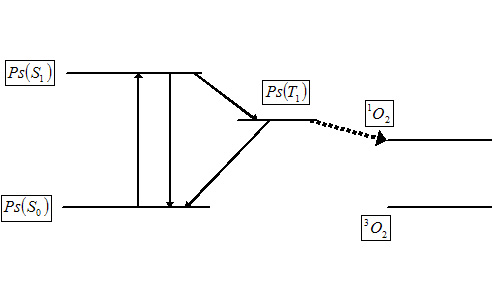
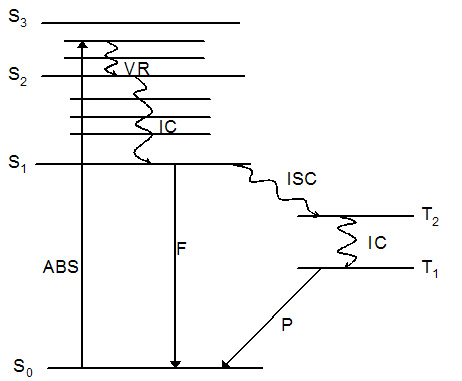
The reason that singlet oxygen is generated in the cells is because of
simple photophysics. Provided that the psoralen possesses an
absorption maximum at a wavelength corresponding with that of the
incident laser light, shining light on a highly colored psoralen
causes excitation to the singlet excited state. The singlet excited
posoralen can decay back to the ground state with release of energy in
the form of fluorescence - enabling identification of tumor tissue or
skin disease. If the singlet state lifetime is suitable (and this is
true for many psoralens) it is possible for the singlet to be
converted into the triplet excited state which is able to transfer
energy to another triplet. One of the very few molecules with a
triplet ground state is dioxygen, which is found in most cells. Energy
transfer therefore takes place to afford highly toxic singlet oxygen
from ground state dioxygen, provided the energy of the triplet excited
state psoralen is higher than that of the product singlet oxygen.
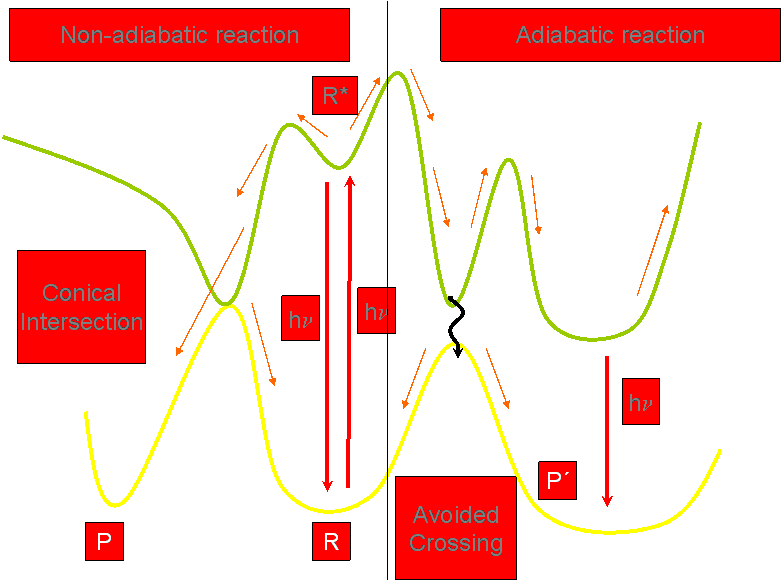
Therefore we can distinguish between two pathways of
photosensitization:
an oxygen-dependent pathway (lipid peroxidation, formation of cross-
links
in membrane proteins, cross-linking of enzyme subunits of oligomeric
proteins) and an oxygen-independent pathway (convalent binding between
furocoumarins and unsaturated fatty acids and lecithins in lipid
membranes, that
they form cross-links with DNA, and that they inactivate enzymes and
ribosomes, dark binding of furocoumarins to lymphocites and skin
cells).
We present here a quantum chemical study of the electronic excited
states and
the photophysics of psoralen molecule, the molecule basis of
furocoumarins
family, in order to interpret its electronic spectra and understand
its photochemotherapeutic effect against some skins diseases such as
psoriasis. In
the future we are going to study the mechanism of the photochemical
reactions involved too. Our study aim to contribute to the
understanding
of
the direct and dynamic mechanisms of antitumoral phototherapeutic
action
and phototherapeutic processes in a general way, and to help to design
new
pharmacons with efective photoinduced properties, trying to optimize
aspects such as the proper absorption or emission energies, energy
transfer to
reactive oxygen, or the formation of adducts without unwanted secondary
efects.
On balance, a good photosensitizer should be amphiphilic (a lot of
psoralens
are poor water-soluble and it is a disadvantage in injected
administration of
the drug because of the the aggregation of the molecules, but their
lipophilic
behaviour is very important too since the intercalative properties
psoralens
towards DNA are due mainly to hydrophobic forces with the adjacement
nu-
cleic acid base pairs), it should form monoadducts and no diadducts
with
DNA to avoid several mutagenic side-efects, it should be effective in
transferring the energy to molecular oxygen (high singlet oxygen
quantum
yield,
i.e. high phosphorescence/fuorescence ratio), its triplet state
must be very
long-lived, its triplet de-excitation energy must lie above the
triplet-singlet excitation energy of oxygen (22.1kcal ·
mol−1), it
should be harmless in
the absence of light (no dark toxicity), it should be easily accesible
(either
by chemical synthesis or isolation from natural sources), and it
should be
removed quickly from the body (nowadays patients who receive this
therapy
become profoundly sensitive to outdoor light for a few weeks period).
And, of
course, the longer wavelength the photosensitizer absorbs, the deeper
electromagnetic radiation penetrates in the body (this fact is very
useful in
cancer
treatment).
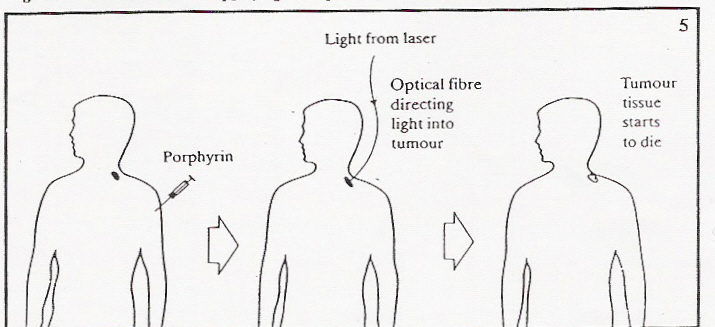
Nowadays PDT is used to treat cancers of the skin or those that are
on, or near, the lining of internal organs. It can be used to treat
cancers in the skin, the head and neck area, the lining of the mouth,
the lining of the lung, the lining of the gullet (esophagus), the
lining of the stomach and the lining of the bladder.
Several different drugs have been developed for PDT, but porfimer
sodium
(Photofrin®) is the only drug that has received official approval for
routine use in patients, and only then for the treatment of certain
cancers. Pilot research studies in patients shown that porfimer sodium
and light can also be useful for psoriasis, but there is a significant
side effect-patients who receive porfimer sodium become profoundly
sensitive to outdoor light for a six to eight weeks period. If
patients must go outdoors, they need to wear protective clothing,
including sunglasses.
The cytotoxic and antitumor actions of PHOTOFRIN are light and
oxygen dependent.
Researchers have investigated the use of laser light and a new drug known as "BPD verteporfin" at UBC for PDT of skin cancer and psoriasis. BPD does not appear to cause prolonged photosensitivity as does porfimer sodium and thus is of potential practical use for psoriasis. To date, BPD and red laser light appear to possess significant anti-psoriasis activity.
The laser light used in PDT can be directed through a fiber-optic
(a very thin glass strand). The fiber-optic is placed close to the
cancer to deliver the proper amount of light. The fiber-optic can be
directed through a bronchoscope into the lungs for the treatment of
lung cancer or through an endoscope into the esophagus for the
treatment of esophageal cancer.
An advantage of PDT is that it causes minimal damage to healthy
tissue. However, because the laser light currently in use cannot pass
through more than about 3 centimeters of tissue (a little more than
one and an eighth inch), PDT is mainly used to treat tumors on or just
under the skin or on the lining of internal organs.
The abnormal blood vessels in the wet form of macular degeneration are
traditionally treated with laser. The thermal energy of the laser
destroys both the blood vessels as well as the surrounding tissue
(retina). Unfortunately, the majority of patients with wet macular
degeneration have abnormal blood vessels located beneath the center of
vision. Thus, laser treatment will destroy the center of vision- the
very thing we want to protect. Photodynamic therapy could allow
selective destruction of abnormal blood vessels without damage to
surrounding tissues. The PDT dye is selectively absorbed by the
abnormally proliferating blood vessels. When exposed to low levels of
light ("non-thermal") the dye is activated and the abnormal blood
vessels are selectively destroyed. PDT therapy is currently being
evaluated in clinical trials. Two drugs are being evaluated:
verteporfin (Vysudine) from CIBA vision and tin ethyl etiopurpurin
(SnET2) from Miravant. Initial results are promising and have shown
at least temporary recovery or preservation of vision in some
patients. FDA approval of the PDT trials await completion of these
studies to assess the overall safety and efficacy of these new
agents.