12. Lewis structure of O3, ozone. Resonance.
1. Compute the total number of valence electrons for all the atoms in the molecule.
Wrong. Check the valence electrons of the three oxygen atoms
|
|
Correct. Each oxygen atom has six electrons in the valence shell.
|
|
Wrong. Check the valence electrons of the three oxygen atoms.
n
|
2. Select the correct structure for O3:
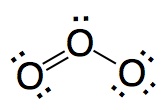
|
Wrong. In this structure the two bonds are different and that is agains the experience.
|
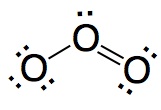
|
Wrong. In this structure the two bonds are different and that is agains the experience.
|
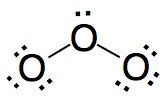
|
Wrong. In this structure the central atom does not reach the eight electron shell.
|
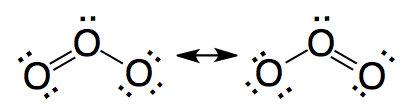
|
Correct. The structure of ozone is described as a resonance of structures.
|
3. How would you describe the resonance of the ozone molecule? Open the animations and select the one you consider more correct.
| Animation1 | Wrong. This animation only shows the stretching and shortening of the bonds.
Such motion is normal in molecules of this shape but is independent from the resonance.
|
| Figura1 | Wrong. The animation shows a mixture of molecules with the bonds exchanged between the atoms
|
| Animation2 | Correct. The electron pair is delocalized to the two atoms. It is a delocalized pi bond.
|