Solutions 2
The following figures represent NaCl solution in water and the interaction of the molecules of the solvent, water, with Na+ (yellow) and Cl-(green) ions.
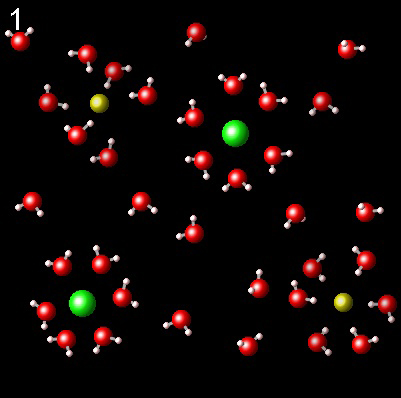 |
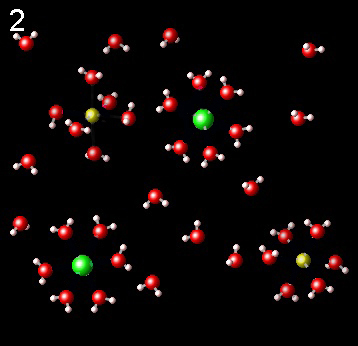 |
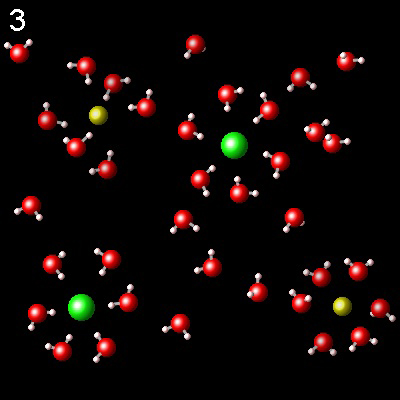 |
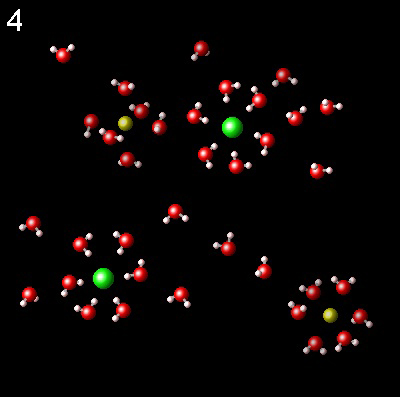 |
Choose the one that best describes the interaction of ions with water
It is not correct. The dipoles of the water
molecule must be correctly oriented toward the ions. Check the polarity of the water molecule.
|
|
It is not correct. The dipoles of the water molecule must
be correctly oriented toward the ions. Check the polarity of the water molecule.
|
|
It is not correct. The dipoles of the water molecule must
be correctly oriented toward the ions. Check the polarity of the water molecule.
|
|
Right. Water molecules are surrounding the
ions in the correct way. Click "Read more".
|