Solutions 3
The following figure represents a solute (S, red) partially dissolved in a solvent (D, white).
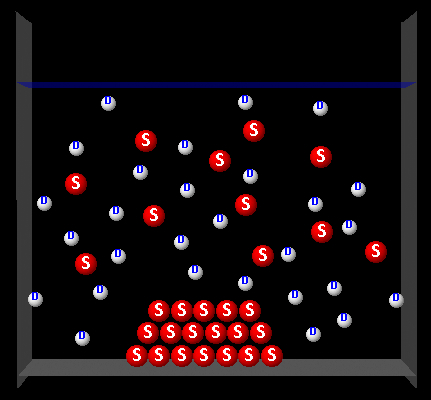 |
Clicking on the following figures can be seen animations that represent four possible dynamic system processes.
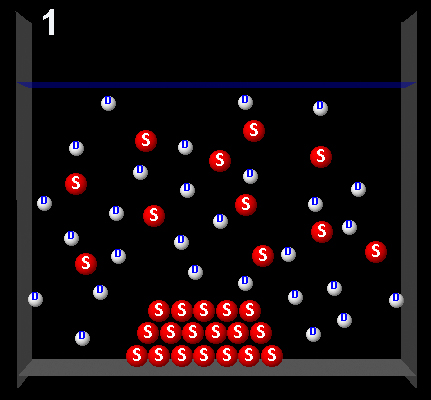 |
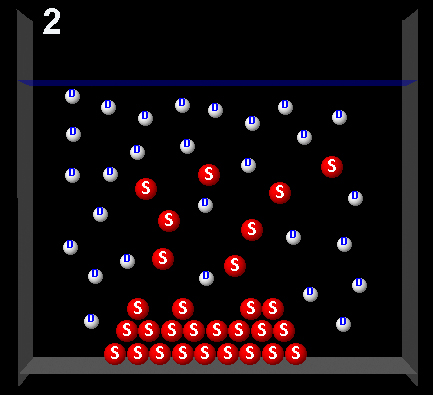 |
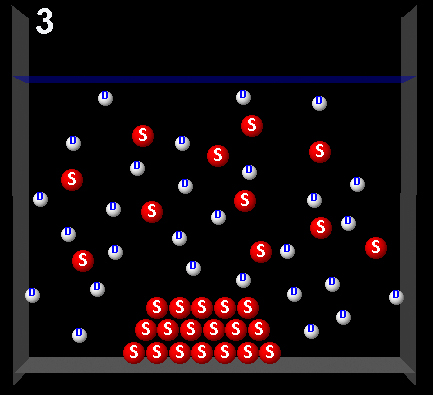 |
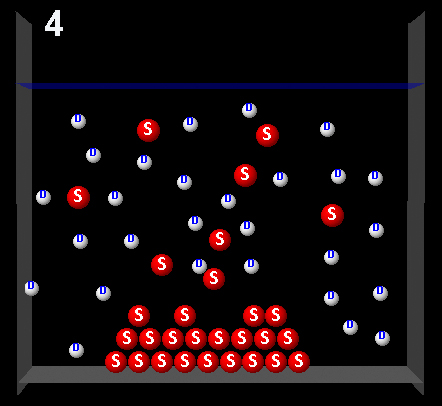 |
After observing the animation choose one that represents a saturated solution.
Wrong. In this animation more solute molecules are passing from the solution to the solid (crystallization)than vice versa. The solution was supersaturated.
|
|
Wrong. In this movie there are more solute molecules passing from the solid to the solution than vice versa. The solution was not saturated.
|
|
Right.This animation shows that during the exchange of solute molecules between the solid and the solution, the amount of solute in solution remains constant. The solution was saturated.
|
|
Wrong. In this movie there is no exchange of solute molecules between the solid and the solution. This situation is not real.
|