Equilibrium 1
The following figure shows the situation of liquid-vapor equlibrio for a given compound at a given temperature.
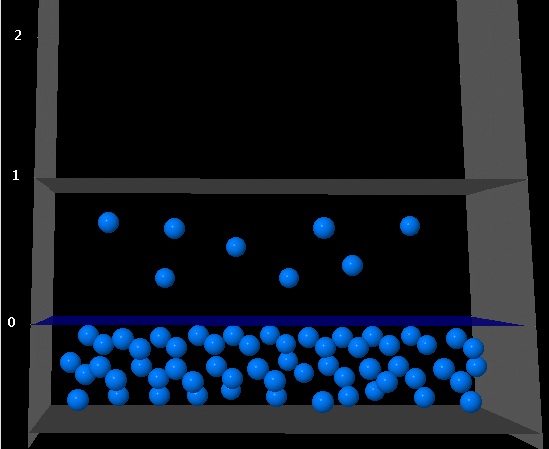 |
If the volume of the vapor phase becomes twice as big, which of the following figures better represents the system once the equilibrium is restored at the same temperature?
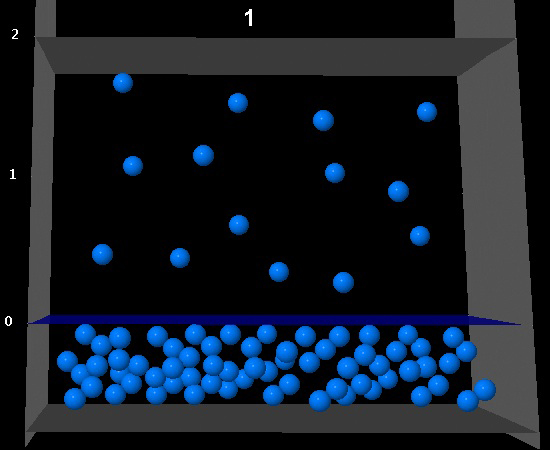 |
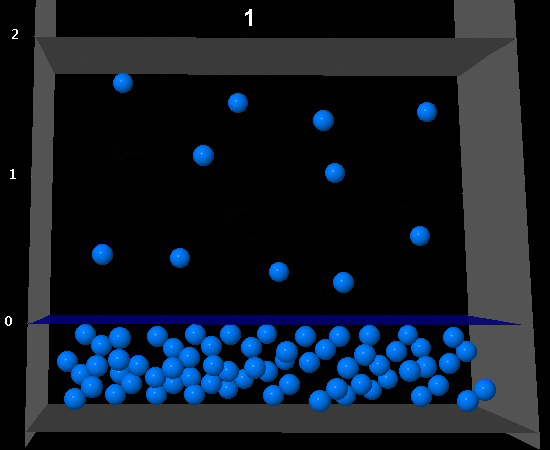 |
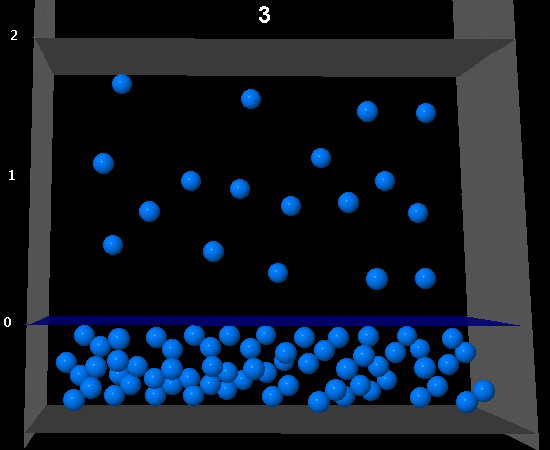 |
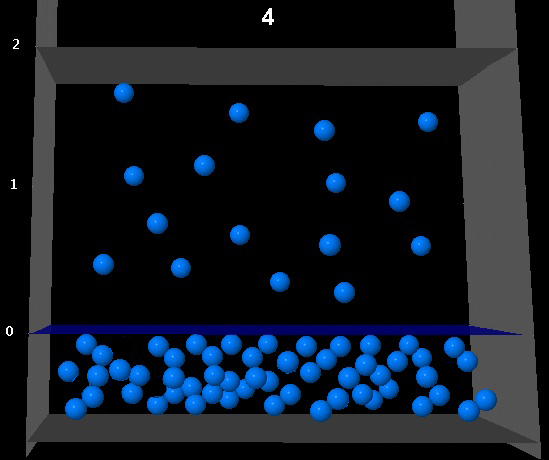 |
Wrong. The camera has doubled but the number of molecules in the vapor phase not.
|
|
Wrong. The volume of the vapor phase has doubled but the number of molecules is the same. That means a vapor pressure half that of the original sample
while the temperature remains constant.
|
|
Wrong. The camera has doubled but the number of molecules in the vapor phase has increased more than twofold.
|
|
Correct. The camera has doubled and the number of molecules in the vapor phase has also doubled. The vapor pressure remains constant.
|