Equilibrium 12
We have an equimolar mixture of two species, A2(g) and B2(g). They react at certain temperature, Ta, according to the following equilibrium reaction: A2(g) + B2(g) ⇄ 2 AB(g)
Considering that Kc= 36 for this reaction at Ta, which of the following diagrams best represents the composition of the mixture once the equilibrium has been reached?
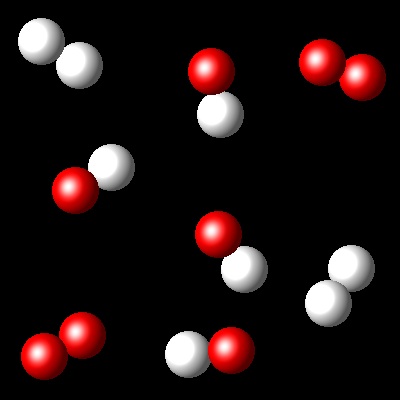 |
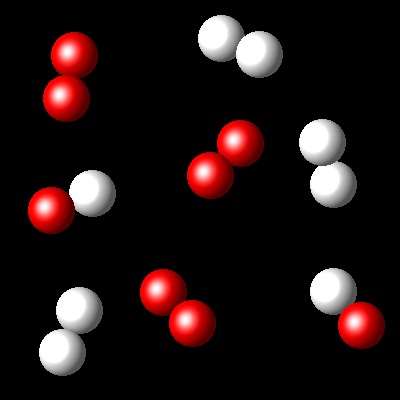 |
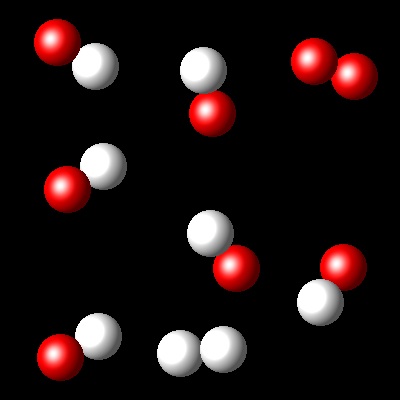 |
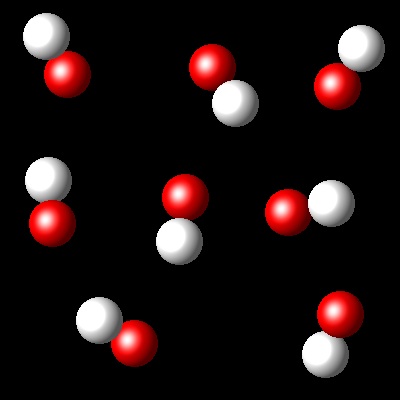 |
Wrong. The reaction progress in this reaction is consistent with a
Kc= 4.
|
|
Wrong. The reaction progress in this reaction
is smaller than what corresponds to a Kc=36.
|
|
Right. The reaction progress corresponds to a Kc=36. See additional text.
|
|
Wrong. In this reaction all reagents have becomed products.
It represent a non equilibrium reaction.
|