Equilibrium 3
These figures show the evolution of a system, at constant temperature gaseous system, from state 1 (non-equilibrium) to state 2 (equlibrium)
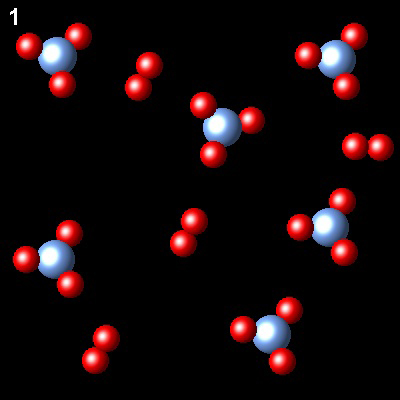 |
 |
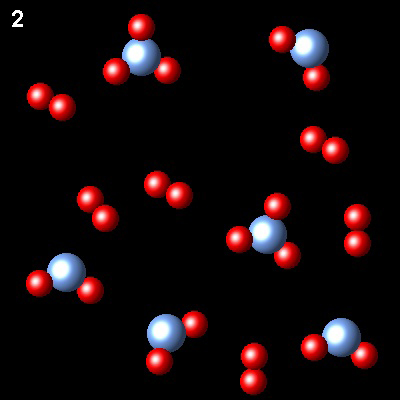 |
What will be the final pressure in the reactor if the initial pressure was Pi.
Wrong. There is a change
in the number of molecules. It must be a change in the pressure.
|
|
Wrong. The numer of molecules
increases. That implies an increase, not a decrease, of the pressure.
|
|
Wrong. The observed increase in
the number of molecules does not justify doubling the pressure
|
|
CORRECTO. In this process
number of gas molecules increased a 20%. The pressure must increase on the same percentage.
|