Equilibrium 2
The figure shows the evolution of a gaseous system containing two types of species, A and B, at constant temperature, from state 1 (non-equilibrium) to state 2 (equilibrium)
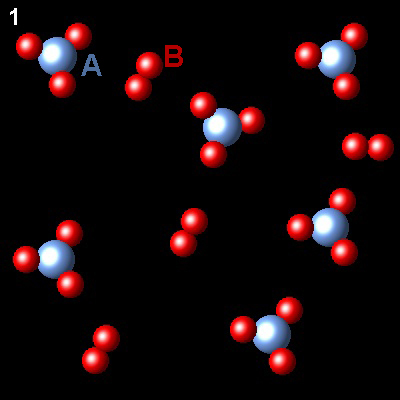 |
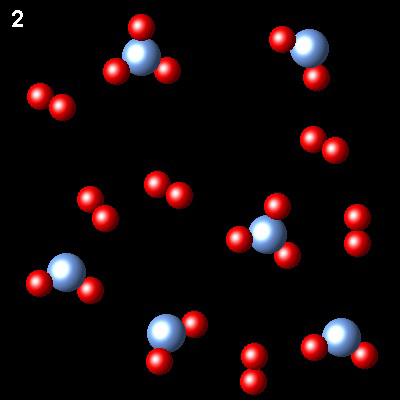 |
If A is represented as blue spheres and B as red spheres, what is the correct chemical equation describing the process?
| AB3⇄AB2+B2 | Wrong. This equation does show the same number of atoms on both sides.
|
| 6AB3⇄4AB2+6B2 | Wrong. This equation does show the same number of atoms on both sides.
|
| 3AB3⇄2AB2+3B2 | Wrong. This equation does
show the same number of atoms on both sides.
|
| 2AB3⇄2AB2+B2 | Right. Two molecules of AB3 are transformed into one of AB2 and B2. This is the balanced chemical equation.
|
Next question: If the process takes place at constant pressure and temperature, what is the final volume of the reactor according to the initial volume Vi?