Gases 12
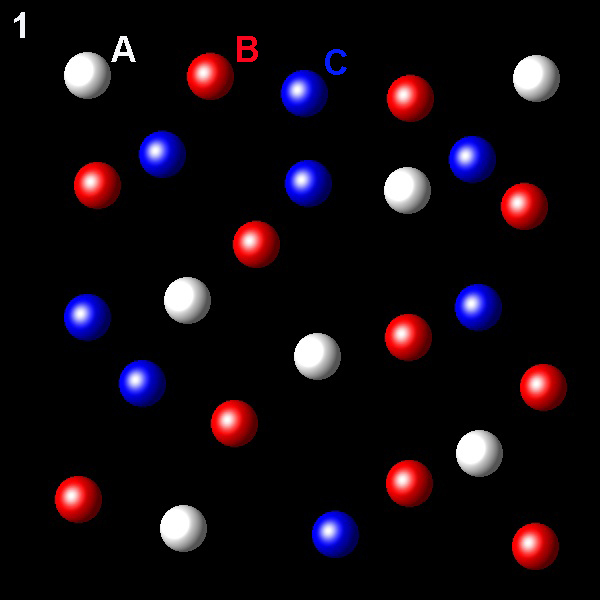
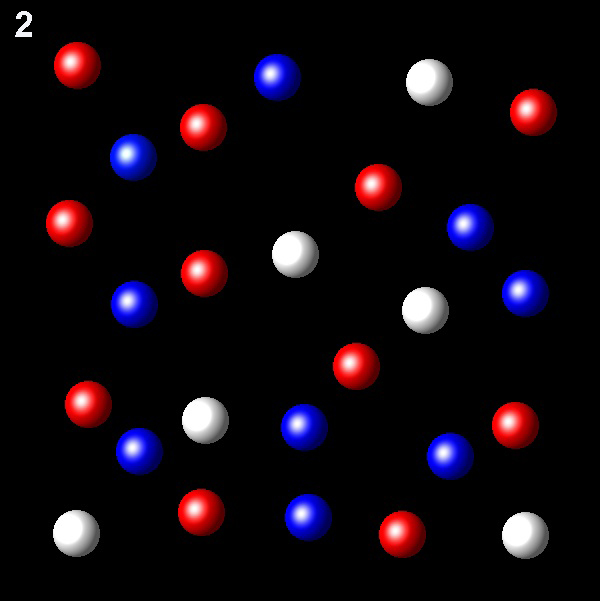
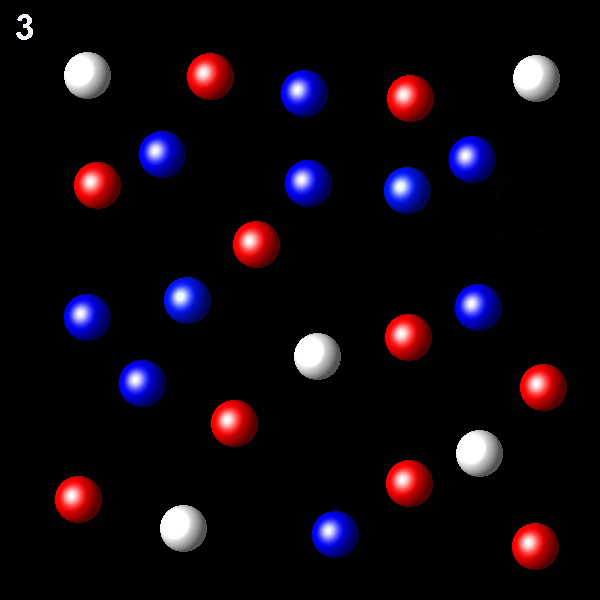
Particles corresponding to three gaseous substances A, B and C, are represented by white, red and blue balls respectively. The following figures show representative portions of the three gaseous substances occupying the same volume and the same temperature.
 |
 |
 |
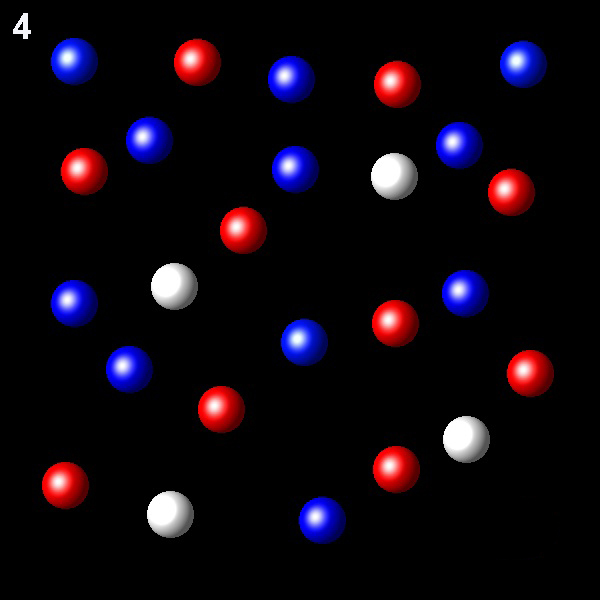 |
Select the figure in which the PB=2PA.
Wrong. So that this condition is met the molar fraction
of B should be twice that of A. This is not true in this figure.
|
|
Wrong. The molar fraction of B should be twice
that of A. In this figure X B=1.5XA.
|
|
Right. In this figure XB= 2XA.
Therefore PB= 2 PA
|
|
Wrong. In this figure XB=2.75XA
|