Gases 11
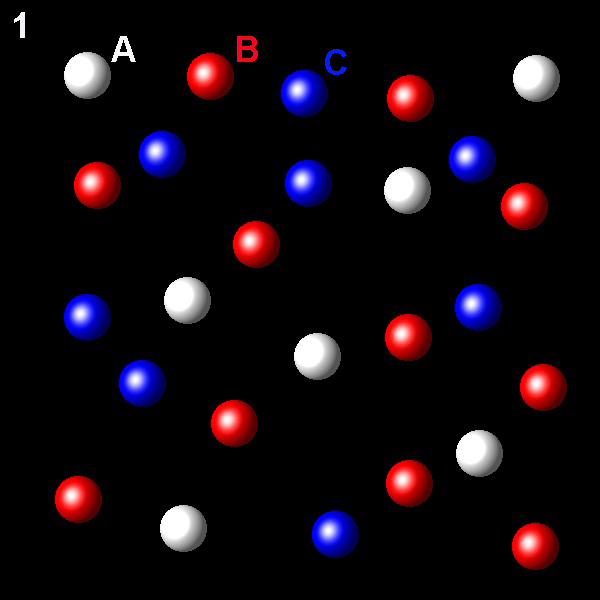
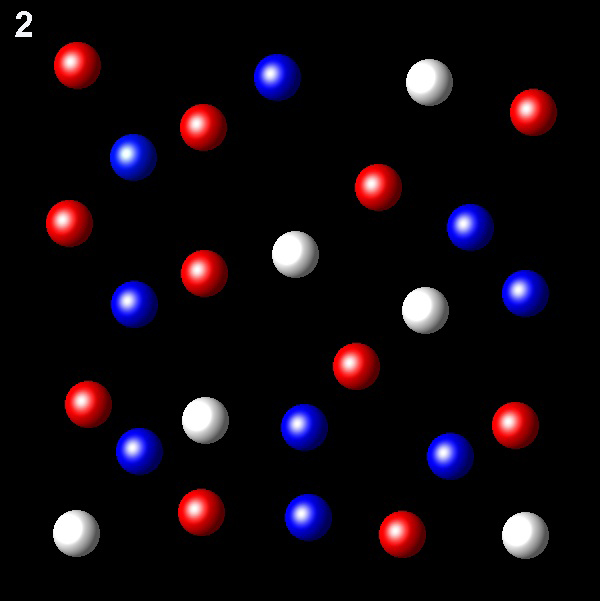
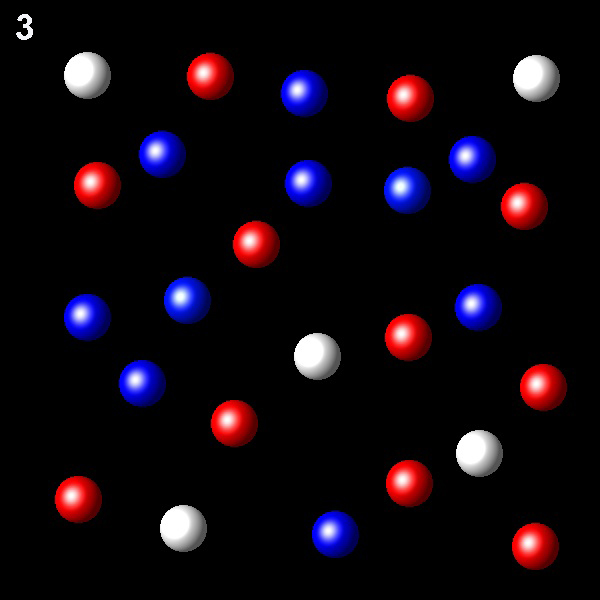
Particles corresponding to three gaseous substances A, B and C, are shown as white, red and blue balls respectively. The following figures show representative portions of the three gaseous substances occupying the same volume at the same temperature.
 |
 |
 |
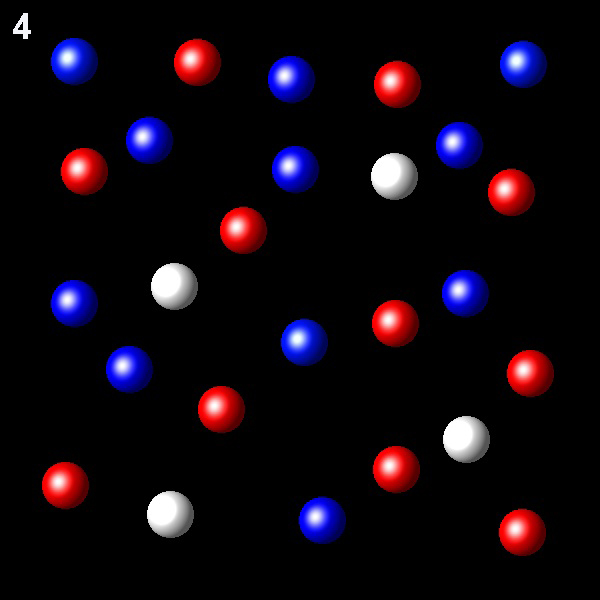 |
Select the figure in which the partial pressure of A is greater.
Right. This figure shows the highest
value of the molar fraction of A, 7/26. Therefore will have the highest partial
pressure of A.
|
|
Wrong. This figure shows the lowest A molar fraction,
6/26.
A. partial
|
|
Incorrecto. Esta figura muestra una
fracción molar de A, 5/26, menor que en otras figuras. Por lo tanto no presenta la mayor presión
parcial de A.
|
|
Wrong. This figure shows a A molar fraction,
5/26, that is not the highest.
|