Gases 10
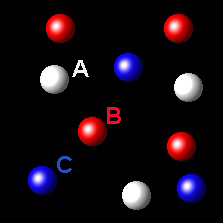
Particles corresponding to three gaseous substances A, B and C, are shown by white, red and blue balls respectively. The following figures show representative portions of the three gaseous substances occupying the same volume at the same temperature.
 |
 |
 |
 |
Which of the figures holds that PA= 2PB
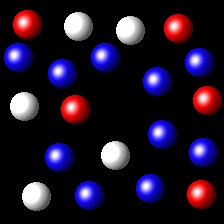
It's not right. In this figure the gases A and
B have molar fractions 3/16 and 4/16 respectively. Therefore their partial pressures
are in the 3: 4 ratio.
|
|
Right. In this figure the gases A and
B have molar fractions of 6/16 and 3/16 respectively. Therefore their partial pressures
are in the 2: 1 ratio as requested.
|
|
Wrong. In this figure, A and B have identical
values of their molar fractions. Therefore the partial pressures are equal.
|
|
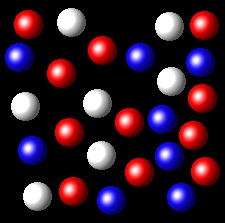
Wrong. In this figure, A has a value of molar
fraction, 6/26, lower than B, 11/26. Therefore pressures do not meet the indicated relationship.
|