Gases 9
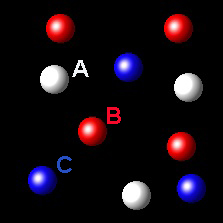
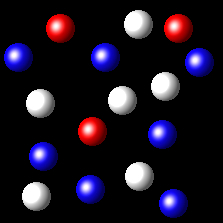
Particles corresponding to three gaseous substances A, B and C, are shown by white, red and blue respectively balls. The following figures show representative portions of the three gaseous substances occupying the same volume at the same temperature.
 |
 |
 |
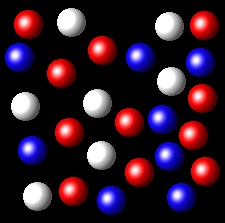 |
Select the figure where the partial pressure of A and C are equal.
Right. In this figure, A and C have
the same molar fraction so their partial pressures are equal.
|
|
Wrong. In this figure, A and C have different
molar fraction so their partial pressures will be different.
|
|
Wrong. In this figure, A and C have different
fraction so their partial pressures will be different.
|
|
Wrong. In this figure, A and C have different
molar fraction so their partial pressures will be different.
|