Gases 8
Particles corresponding to three gaseous substances A, B and C, are represented by white, red and blue balls respectively. The following figures show representative portions of the three gaseous substances occupying the same volume at the same temperature.
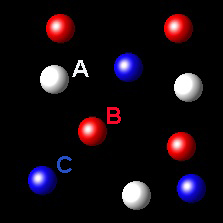 |
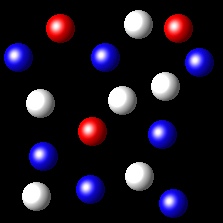 |
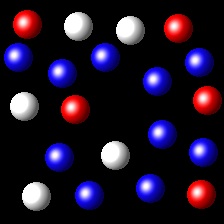 |
 |
Select the figure in which the partial pressure of A is greater.
Wrong. The sample with the highest number of
molecules of A will have greater partial pressure of A. This one is not that case.
|
|
Wrong. The sample with the highest number of
molecules of A will have greater partial pressure of A. This one is not that case
|
|
Wrong. The sample with the biggest number of
molecules of A will have greater partial pressure of A. This one is not that case
|
|
Right. In this figure the number of A molecules is the greatest of all.
Therefore the partial pressure of A will be the largest.
|