Reaction 4
The figure on the left represents one set chlorine (green) and hydrogen (white) molecules. The figure on the right shows the result of the reaction between these species.
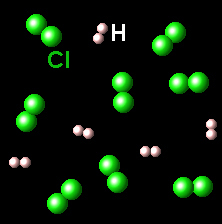 |
 |
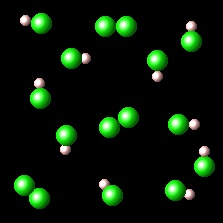 |
Which of the following equations best represents the chemical reaction taking place?
| 10 H + 10 Cl → 10 HCl | WRONG. The two species
are diatomic molecules. The lowest stoichiometric coefficients should be used.
|
| H + Cl → HCl | Wrong. The two species
are diatomic molecules.
|
| H2 + Cl2 → 2 HCl | Right. One molecule of hydrogen reacts
with one molecule of chlorine to form two molecules of HCl.
|
| 5 H2 + 8 Cl2 → 10 HCl + 3 Cl2 | Wrong. Reagents not correspond to what is shown in the Figure.
|