Reaction 5
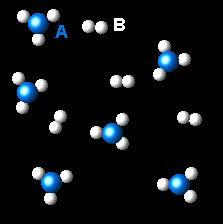
In the figure it is shown the evolution of a gaseous system at constant temperature from the initial state(left) to the final state (right).
 |
 |
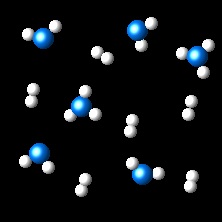 |
If the atoms A are represented as blue spheres and atoms B by white spheres, what is the chemical equation for the process?
| AB3 ⇄ AB2 + B2 | Wrong. This chemical
equation is not correct. The number of B atoms doas not remain contant in both sides
|
| 6AB3 ⇄ 4AB2 + 6B2 | Wrong. This chemical
equation is not correct. The number of atoms increase.
|
| 4AB3 ⇄ 4AB2 + 2B2 | Wrong. This chemical
equation is not correct. It does not use the correct stoichiometric coefficients.
| .
| 2AB3 ⇄ 2AB2 + B2 | Right. The
chemical equation is correct. Two molecules of AB3 are transformed in two of AB2 and one of B2.
|