METHODS
Provenance and collection of materials
Most of the surveys on extant
polycystine radiolarians published to date are based on samples
of their skeletons preserved in the surface sediments, rather
than on plankton samples. Sediment samples have some advantages
over water-column materials, but also several important
shortcomings (see Sedimentary vs.
water-column materials).
A variety of sediment coring and
grabbing devices have been used throughout the years for analyses
of the polycystines from the upper centimeters of the sediments (Kennett 1982). Gravity and Kasten corers are among the
simplest, permitting retrieval of up to a few meters of sediment
at a time from practically any depth. Piston corers have been
used widely due to their ability to recover long sedimentary
sequences, up to 20-30 m in length. However, all these devices
tend to disturb the sediments, especially the uppermost layer
which is of particular importance for the analysis of Recent
assemblages. Box corers (rectangular, shallow, ca. 1 m coring
boxes which ensure complete closure of water-flow passages after
sampling and before leaving the seabed, thus minimizing sample
washout during ascent) are preferable for retrieval of the top
layer of the sediments. However, the fact that box core samples
usually lack the thin uppermost phytodetrital film characteristic
of most sediments (Billett et al. 1983) suggested that the bow-wave of the device
is strong enough as to wash away any mobile particles before
hitting the bottom. Multicorers, an arrangement of several short
coring tubes mounted on a rigid frame seem to overcome this
problem successfully as they have been shown to collect
phytodetritus, as well as significantly higher numbers of
macrobenthic specimens than box corers (Bett et al. 1994).
Plankton samples for radiolarian
studies are usually collected with nets. However, this group, as
well as a few other microzooplanktonic taxa, pose serious
methodological difficulties. Indeed, they are too small (around
20-30 to 300 µm) to collect effectively with standard
zooplanktonic nets (100 to 300 µm in pore size), yet too scarce
in most areas to yield adequate catches with water-bottles or
low-powered pumps. Thus, fine-meshed nets have to be employed,
which significantly complicates not only the concentration of the
radiolarians (due to the concomitant retrieval of other
organisms, some of which, like the diatoms, cannot be fractioned
out later; see Swanberg and Eide 1992), but also because net clogging jeopardizes
subsequent estimations of the volume of water filtered (Tranter and Smith 1968; Boltovskoy 1981b). In order to avoid clogging by smaller
particles, thus ensuring better estimates of the volume of water
filtered and larger sample-sizes, meshes ranging between 60 and
up to 100 µm are traditionally used for polycystine studies in
the water-column. It should be stressed, however, that both
absolute quantitative estimates of radiolarian abundance, and the
proportions of at least some species and developmental stages may
be seriously biased in these collections: Boltovskoy et al. (1993a) reported that in sediment trap materials
from the tropical Atlantic shells below 40-60 µm represent
roughly 50% of the overall polycystine fauna.
Estimates of radiolarian abundances
in the water-column must be performed with flow-metered nets;
clogging of the meshes, in particular of those with small pores,
makes assessment of the volume of water filtered based on
distance towed and mouth diameter extremely unreliable (Tranter and Smith 1968; Boltovskoy 1981a, b). Thus, whenever unflowmetered nets are
employed, such as those derived from Tucker's (1951) opening-closing mechanism (e.g., the
Multinet, based on Bé's 1962, design; the MOCNESS, Wiebe et al. 1976; the RMT 1+8, Baker et al. 1973), it is strongly recommended that
evaluation of radiolarian concentrations be avoided (species
proportions, on the other hand, are in principle unaffected in
these samples).
For assessment of the delicate
colonial forms, as well as for studies of feeding, growth,
metabolism, etc. of live individuals, specimens are collected by
divers (e.g., Swanberg 1979), or by means of very short and slow
plankton tows, thus ensuring a better preservation of the
protists (Matsuoka 1992).
Sediment trap techniques have
undergone major improvements in the last years, thus constituting
a very useful tool for the collection of polycystine materials (US GOFS 1989; Lange and Boltovskoy 1995). Simple sediment traps consist of a
concentrating cone or funnel which tapers into a collecting jar;
the array, which can have either one or several traps, is moored
to the bottom or drifts with the current suspended from a buoy at
the surface. Time-series models are deployed at different oceanic
locations for periods up to a year or more, and are provided with
a mechanism which replaces the collecting cup at predetermined
intervals thus yielding a detailed record of the changes in the
amount and type of flux throughout several seasons (Honjo and Doherty 1988; Lange and Boltovskoy 1995).
Sediment-trap materials have some
important advantages over planktonic collections. Sample-size is
usually much larger in sediment traps than in plankton nets, with
fluxes as high as 200,000 shells/m2/day having been
recorded in the equatorial Atlantic (Boltovskoy et al. 1996; see also table 3 in Boltovskoy et al. 1993a). Seasonal plankton collections are
composed of a sequence of snapshots which represent but an
insignificant proportion of the total time elapsed between tows,
and may therefore not only under- or overestimate mean protist
abundances (e.g., Bé et al. 1985), but also yield "atypical"
specific assemblages. Time-series sediment trap samples, on the
other hand, integrate over preselected depth and time ranges,
thus averaging the overlying plankton over restricted periods
which yield adequate chronological resolution to allow
pinpointing the relative importance of limited offsets of the
yearly cycle. Furthermore, since seasonal variations in total
mass flux are usually closely coupled with primary production in
the upper mixed layer (Honjo et al. 1982, 1988; Deuser et al. 1983, 1990; Wefer 1989), comparison of total flux vs. radiolarian
numbers and specific makeup can furnish first hand information on
indicators (and paleoindicators) of the biological productivity
of the associated water masses.
 Sediment trap materials, however, also have
some shortcomings. Because of limitations associated with the
hydrodynamic properties of particle accumulation in the traps,
these devices are most effective when deployed at depths in
excess of 500-700 m (US GOFS 1989; Lange and Boltovskoy 1995). As a result, they integrate the flux from
several biologically dissimilar layers (e.g., Kling 1979; Kling and Boltovskoy 1995). Furthermore, sinking skeletons
intercepted at these depths may not adequately reflect their
standing stocks at the surface, nor their specific composition. Boltovskoy and Alder
(1992) concluded that, in
the Weddell Sea, over 90% of the polycystines that inhabit the
upper 400 m are destroyed (probably due to fragmentation by
grazing) before reaching 400-900 m of depth.
Sediment trap materials, however, also have
some shortcomings. Because of limitations associated with the
hydrodynamic properties of particle accumulation in the traps,
these devices are most effective when deployed at depths in
excess of 500-700 m (US GOFS 1989; Lange and Boltovskoy 1995). As a result, they integrate the flux from
several biologically dissimilar layers (e.g., Kling 1979; Kling and Boltovskoy 1995). Furthermore, sinking skeletons
intercepted at these depths may not adequately reflect their
standing stocks at the surface, nor their specific composition. Boltovskoy and Alder
(1992) concluded that, in
the Weddell Sea, over 90% of the polycystines that inhabit the
upper 400 m are destroyed (probably due to fragmentation by
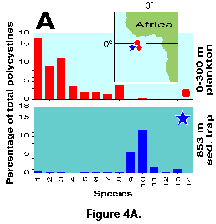
grazing) before reaching 400-900 m of depth. 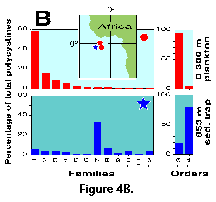 Subsurface advection of shells produced at
higher latitudes and integration of low protist abundances over
large depth intervals may be responsible for the fact that, in
the eastern equatorial Atlantic, polycystine assemblage
compositions recorded in plankton samples at 0-300 m are totally
different from those recovered in traps at 800-2000 m (Boltovskoy et al. 1995; Figure 4A, 4B).
Subsurface advection of shells produced at
higher latitudes and integration of low protist abundances over
large depth intervals may be responsible for the fact that, in
the eastern equatorial Atlantic, polycystine assemblage
compositions recorded in plankton samples at 0-300 m are totally
different from those recovered in traps at 800-2000 m (Boltovskoy et al. 1995; Figure 4A, 4B).
It should be borne in mind that the
yields of sediment trap samples are not amenable to direct
comparisons with those of plankton samples: while the former are
an expression of the downward flux, which in turn is associated
with productivity and preservation, quantitative plankton samples
give information on standing stock only. Hence, compositional
differences may not only reflect advection, destruction by
grazing, etc., but also biological traits of the species
considered. Thus, a scarce species with high reproduction,
mortality and output rates may be rare in the plankton but
abundant in the underlying sediment trap (Kling and Boltovskoy 1995; Boltovskoy et al. 1995).
As with other zooplanktonic groups,
analyses of radiolarian vertical distribution patterns are
usually performed with the aid of vertically stratified plankton
tows (e.g., Renz 1976; Kling 1979; Dworetzky and Morley 1987; Kling and Boltovskoy 1995; Abelmann and Gowing 1997). However, because their identification is
based on the siliceous skeleton which preserves after the death
of the cell, in order to discriminate live vs. dead protists in
the subsurface layers the cytoplasm is often stained with Rose
Bengal, Sudan black B, or eosin (Petrushevskaya 1971b; Swanberg and Bjųrklund
1986; Abelmann and Gowing 1997). Although this technique can furnish some
clues on the living depth ranges of the species, it does not
provide unequivocal information because of uncertainties
associated with the speed of decomposition of the protists'
cytoplasm. Boltovskoy and Lena
(1970), for example,
concluded that specimens of several planktonic foraminifera still
contained protoplasm in their shell 98 days after death. Bernhard (1988) compared estimates of the proportions of
presumably live benthic Foraminifera as indicated by Rose Bengal
and Sudan black B staining and by ATP assay, concluding that
stained protoplasm was present in individuals up to four weeks
after actual death of the cell. These lapses are significantly
longer than the time it takes a radiolarian shell to reach the
sea-floor (Takahashi and Honjo 1983).
Unless special cytological studies
are required (e.g., Petrushevskaya 1986), plankton and sediment trap samples can be
preserved in 4-5% formaldehyde; the addition of picric acid to
the solution enhances the preservation of the colonies, yet
acidification should be avoided if the calcareous plankton is to
be saved from dissolution.
Sample preparation and analysis
The following section offers some
general comments on the preparation of whole samples for routine
counting and identification procedures. It does not review the
methods involved in special cytological and ultrastructural
studies (see Anderson 1983a, for a review of these topics), as well as
those used for detailed taxonomic work, which can involve
thin-sectioning, etching and polishing, etc. (Riedel and Sanfilippo
1977; Boltovskoy et al. 1983; Petrushevskaya 1986).
Pelagic surface sediments are
usually clean enough as to require little treatment before
preparation of the slides. Elimination of the organic matter and
disaggregation of the materials is achieved by boiling the sample
(5-10 g) for a few minutes in a beaker with water to which
hydrogen peroxyde (10%, 300 ml per liter) and tetrasodium
pyrophosphate (10 g per liter) have been added. Disaggregation,
cleaning and removal of clay coatings and infilling particles can
be aided by treating the sample in a gentle ultrasonic bath. For
further disaggregation of heavily indurated sediments various
products, such as kerosene, paint thinner, or ammonia can be
helpful (the sediment is dried, soaked in the solvent, and then
immersed in water, upon which disaggregation usually occurs
rapidly). If calcareous material is abundant it can be removed
with a few drops of hydrochloric acid (after eliminating the
hydrogen peroxyde by wet-sieving). The resulting clean material
is then sieved with abundant water in order to eliminate the
reagents and smaller particles. The mesh size used depends on the
aims of the study; most surveys routinely employ 40-60
µm-meshes, yet these, as described above, miss many of the
smaller species, as well as most developing forms. If precise
abundance estimates are sought, mesh openings around 15 to 20 µm
should be employed, although these will retain large numbers of
unidentifiable skeletal fragments, as well as non-radiolarian
material (especially diatoms), which can make subsequent
observation more laborious. The clean residue in the sieve is
pipetted onto glass microscope slides, dried, and soaked with a
few drops of xylene; before the xylene has evaporated the
mounting medium is added and covered with a cover glass. Canada
Balsam is most often used for these preparations, although it
takes longer to harden than some other synthetic materials,
commercially known as Norland, Pleurax, Hyrax or Depex.
Moore (1973) proposed a convenient method which allows
quantification of the number of radiolarian shells per unit
weight of sediment. Before processing as described above, the
sample is dried and weighed. This weighed sediment is then
cleaned and sieved, and all the resulting residue is poured into
a large (e.g., 5 l) beaker full of distilled water, on the bottom
of which one or two cover gasses have been positioned. The water
with the sediment in the beaker is then thoroughly stirred
(avoiding rotational motion, which will result in centrifugal
fractionation) for achieving a random distribution of the
particles, and the sediment is allowed to settle. With the aid of
a siphon all but 30-50 mm of water are removed, and the remainder
is evaporated with an overhead infrared lamp. When the surface of
the cover glasses is dry they are removed from the beaker and
mounted as described above. The slide thus prepared will contain
a fraction of the radiolarian shells present in the original
sample, this fraction being equivalent to the proportion that the
surface of the cover glass makes of that of the surface of the
bottom of the beaker.
Preparation of plankton and
sediment trap samples is somewhat more labororious due to the
large amounts of organic material they contain. When both
absolute radiolarian concentrations and specific inventories are
sought, it is recommended that counting be performed separately
from the identifications. Polycystines can be counted (although
not identified) in whole, unprocessed samples in counting
chambers under the inverted microscope (Hasle 1978; Boltovskoy 1981c; Villafańe and Reid 1995). Subsequently, either the entire sample or
a subsample can be treated in order to eliminate all organic
matter leaving the clean siliceous skeletons that will be mounted
as described above for sedimentary materials. It should be born
in mind, however, that radiolarian cells are often very difficult
to recognize in preserved, unprocessed plankton samples. The
siliceous skeleton, usually the most conspicuous distinguishing
feature, is obscured by the cytoplasm to such an extent that
radiolarians are easily confused with other planktonic protists,
fecal pellets, eggs, various organic aggregates, debris, etc.
Adding a few drops of hydrogen peroxide and/or hydrochloric acid,
which slowly digest the organic matter, and comparing the dubious
particles before and after treatment can greatly help to pinpoint
radiolarian cells (Alder, personal commun. 1997).
Several different methods have been
used for eliminating organic material from water-column samples,
including high- and low-temperature ashing, oxidizing with
hydrogen peroxide and/or ultraviolet light, etc. (see review in Boltovskoy et al. 1983). One of the most widespread, however, is
that proposed by Simonsen (1974) for cleaning diatom frustules. The plankton
sample is rinsed with abundant fresh water (wet-sieving), and
placed in a beaker to which an equal volume of saturated KMnO4
is added; it is then left for 24 hs. A volume of concentrated HCl
equivalent to that already contained in the beaker is
subsequently added to the sample; the dark brown liquid is gently
heated until it becomes transparent or light yellow. Once the
sample has cooled, it is sieved again thoroughly with fresh water
and rinsed with distilled water. The residue is pipetted onto
microscope glass slides as described above.
 Analysis of the specimens is best performed
in mounted slides, which by transparency permits observing the
internal structures (such as medullary shells, spiral structures,
etc.), and the wall-thickness. In addition, slight variations in
the depth of field allow one to determine whether a shell of
circular outline is a disc (in which case most of the surface is
in focus simultaneously), or a sphere (either the central part or
the periphery are in focus).
Analysis of the specimens is best performed
in mounted slides, which by transparency permits observing the
internal structures (such as medullary shells, spiral structures,
etc.), and the wall-thickness. In addition, slight variations in
the depth of field allow one to determine whether a shell of
circular outline is a disc (in which case most of the surface is
in focus simultaneously), or a sphere (either the central part or
the periphery are in focus). 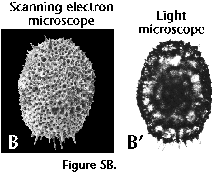 Photographs taken in the light microscope
have the advantage of being readily comparable to mounted
specimens. The scanning electron microscope (SEM), on the other
hand, is especially suitable for analyzing the surface
morphology, but only in specimens with large openings in the
outermost shell, or in those partially broken, can internal
structures be observed. SEM photographs produce very appealing
results, but their comparison with routine collections mounted in
slides is tricky (see Figure 5A, 5B, 5C).
Photographs taken in the light microscope
have the advantage of being readily comparable to mounted
specimens. The scanning electron microscope (SEM), on the other
hand, is especially suitable for analyzing the surface
morphology, but only in specimens with large openings in the
outermost shell, or in those partially broken, can internal
structures be observed. SEM photographs produce very appealing
results, but their comparison with routine collections mounted in
slides is tricky (see Figure 5A, 5B, 5C). 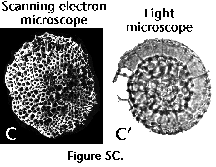 Ideally, both techniques should complement
each other (Boltovskoy et al. 1983, described a method which allows performing
light and SEM observations and photographs of the same
radiolarian specimens).
Ideally, both techniques should complement
each other (Boltovskoy et al. 1983, described a method which allows performing
light and SEM observations and photographs of the same
radiolarian specimens).
Assessment of radiolarian
species-specific absolute and relative abundances are based on
identifications and counts. Since any given slide often contains
thousands of polycystine shells, the researcher is forced to
decide how many specimens should be identified and counted in
order to achieve an adequate estimate of overall numbers and
species proportions. Several methods have been proposed for the
assessment of bias in sample-based particle counts (see reviews
in Venrick 1978, 1995; Frontier 1981), and in the appraisal of species
proportions (Patterson and Fishbein
1989; Buzas 1990). Patterson and Fishbein
(1989) concluded that for
species representing >50% of the overall taxocoenosis at least
50 specimens should be counted in order to achieve reliable
percentage data, 300 counts for species which comprise
approximately 10% of a sample, 500-1000 counts for species that
make up 5%, and counts of several thousands for those that
comprise 1%. Unfortunately, in the case of the polycystines these
efforts are unrealistic because in any given sample containing
100-150 species only one-three are above 10%, and 70-90 occur at
levels below 1% (see "Geographic and vertical distribution"). In terms of the amount of
information attained, it is more profitable to analyze more
samples at a lower resolution, than to examine fewer sites at
these statistically more reliable levels. Thus, in practice
proportions are estimated in bulk, regardless of the individual
species abundances, usually scanning 300-600 specimens per
sample. It is common practice to identify the first 300-600
individuals on the slide, and then check the rest of the slide or
slides for the given sample in order to account for the rarer
taxa. The relative abundances of the latter are estimated
approximately, and they are usually excluded from subsequent
general numerical analyses (e.g., multivariate techniques, such
as cluster and factor analysis) because of the uncertainties
associated with their assumed absences. It should be stressed,
however, that the counting effort necessary for reliable
estimates of the fractional abundance of the rare species is
inversely proportional to the equitability of the assemblage.
Thus, when the sample is strongly dominated by a single or only a
few taxa, such as in polar areas, chances of recording the rare
polycystines in random sequential counts are low because the
observer repeatedly hits the dominant species. On the contrary,
as equitability increases so does the probability of logging a so
far unrecorded species with every new specimen scanned.
 Sediment trap materials, however, also have
some shortcomings. Because of limitations associated with the
hydrodynamic properties of particle accumulation in the traps,
these devices are most effective when deployed at depths in
excess of 500-700 m (US GOFS 1989; Lange and Boltovskoy 1995). As a result, they integrate the flux from
several biologically dissimilar layers (e.g., Kling 1979; Kling and Boltovskoy 1995). Furthermore, sinking skeletons
intercepted at these depths may not adequately reflect their
standing stocks at the surface, nor their specific composition. Boltovskoy and Alder
(1992) concluded that, in
the Weddell Sea, over 90% of the polycystines that inhabit the
upper 400 m are destroyed (probably due to fragmentation by
grazing) before reaching 400-900 m of depth.
Sediment trap materials, however, also have
some shortcomings. Because of limitations associated with the
hydrodynamic properties of particle accumulation in the traps,
these devices are most effective when deployed at depths in
excess of 500-700 m (US GOFS 1989; Lange and Boltovskoy 1995). As a result, they integrate the flux from
several biologically dissimilar layers (e.g., Kling 1979; Kling and Boltovskoy 1995). Furthermore, sinking skeletons
intercepted at these depths may not adequately reflect their
standing stocks at the surface, nor their specific composition. Boltovskoy and Alder
(1992) concluded that, in
the Weddell Sea, over 90% of the polycystines that inhabit the
upper 400 m are destroyed (probably due to fragmentation by
grazing) before reaching 400-900 m of depth.  Subsurface advection of shells produced at
higher latitudes and integration of low protist abundances over
large depth intervals may be responsible for the fact that, in
the eastern equatorial Atlantic, polycystine assemblage
compositions recorded in plankton samples at 0-300 m are totally
different from those recovered in traps at 800-2000 m (Boltovskoy et al. 1995; Figure 4A, 4B).
Subsurface advection of shells produced at
higher latitudes and integration of low protist abundances over
large depth intervals may be responsible for the fact that, in
the eastern equatorial Atlantic, polycystine assemblage
compositions recorded in plankton samples at 0-300 m are totally
different from those recovered in traps at 800-2000 m (Boltovskoy et al. 1995; Figure 4A, 4B). Analysis of the specimens is best performed
in mounted slides, which by transparency permits observing the
internal structures (such as medullary shells, spiral structures,
etc.), and the wall-thickness. In addition, slight variations in
the depth of field allow one to determine whether a shell of
circular outline is a disc (in which case most of the surface is
in focus simultaneously), or a sphere (either the central part or
the periphery are in focus).
Analysis of the specimens is best performed
in mounted slides, which by transparency permits observing the
internal structures (such as medullary shells, spiral structures,
etc.), and the wall-thickness. In addition, slight variations in
the depth of field allow one to determine whether a shell of
circular outline is a disc (in which case most of the surface is
in focus simultaneously), or a sphere (either the central part or
the periphery are in focus).  Photographs taken in the light microscope
have the advantage of being readily comparable to mounted
specimens. The scanning electron microscope (SEM), on the other
hand, is especially suitable for analyzing the surface
morphology, but only in specimens with large openings in the
outermost shell, or in those partially broken, can internal
structures be observed. SEM photographs produce very appealing
results, but their comparison with routine collections mounted in
slides is tricky (see
Photographs taken in the light microscope
have the advantage of being readily comparable to mounted
specimens. The scanning electron microscope (SEM), on the other
hand, is especially suitable for analyzing the surface
morphology, but only in specimens with large openings in the
outermost shell, or in those partially broken, can internal
structures be observed. SEM photographs produce very appealing
results, but their comparison with routine collections mounted in
slides is tricky (see  Ideally, both techniques should complement
each other (
Ideally, both techniques should complement
each other (