Equilibrium 10
The following figures represent portions of the initial and final states corresponding to the reaction:
H2(g) + CO2 ⇄ H2O(g) + CO(g) at a temperature of 2000 K.
 |
 |
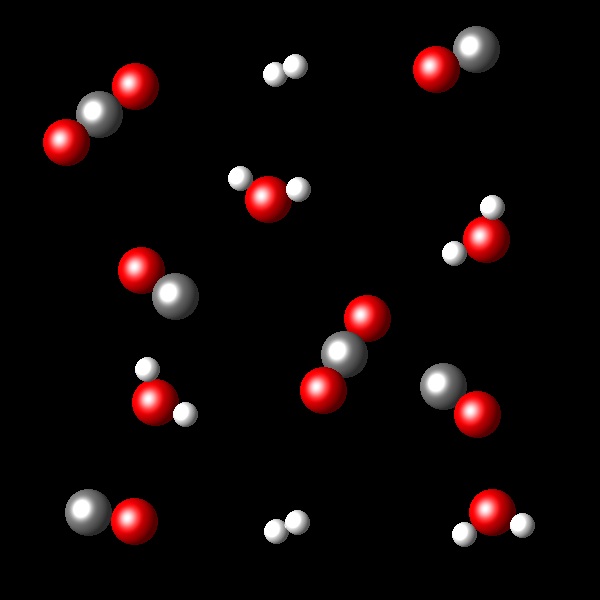 |
Considering the transformation represented calculate the equilibrium constant Kc for this process.
Wrong.This value would mean that in the final state
concentrations of reactants and products were equal. Such a thing does not happen.
|
|
Right. In the final state the concentration of
products is twice that of the reactants giving a Kc=4.
|
|
Wrong. This value is very small and represents a
very low conversion.
|
|
Wrong. The conversion is greater than what represents a Kc=2.
|