Equilibrium 9
Process A(g) → B(g) is exothermic; ΔH<0. When this reaction is performed in a system closed reaches an equilibrium state A(g) ⇄ B(g) which can be represented as follows:
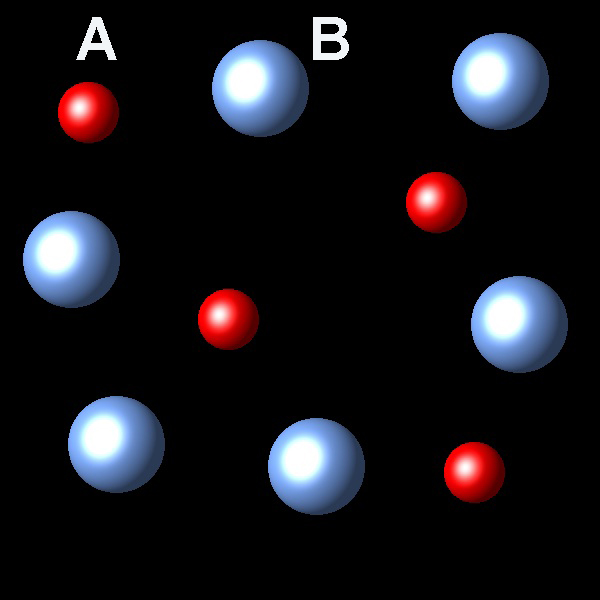 |
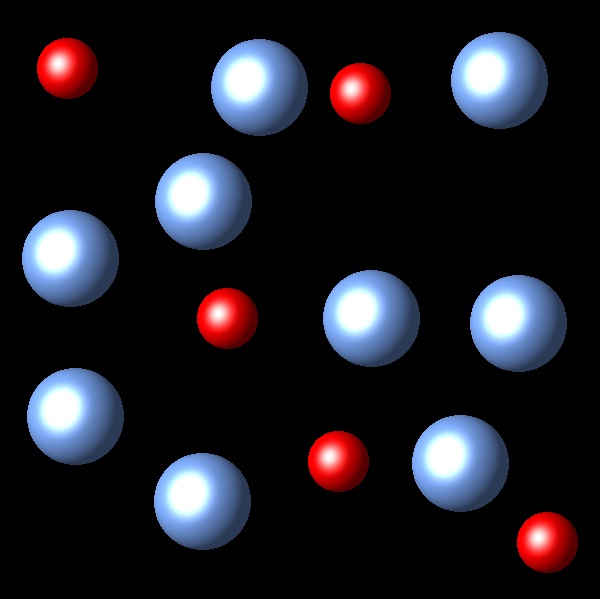
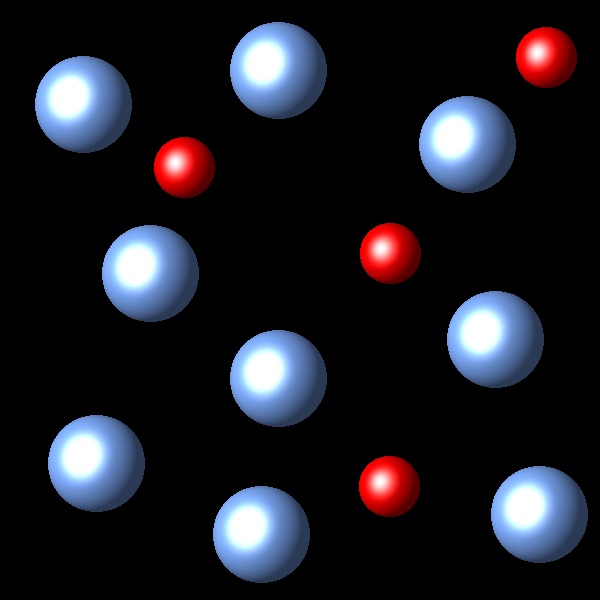
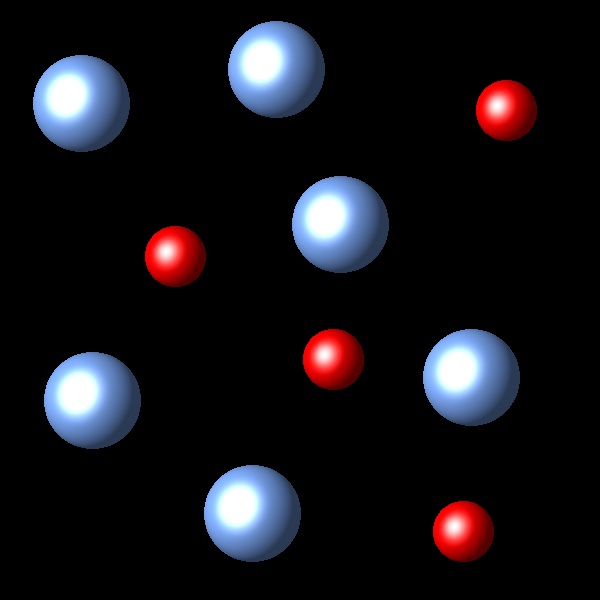
If we increase the pressure to this system in equilibrium which of the following representations best describes the new situation of equilibrium system?
Wrong
|
Wrong
|
Right
|
Wrong
|
 |
 |
 |
 |
The change of volume has not been represented in these figures.