Equilibrium 8
Process A(g) → B(g) is exothermic; ΔH<0. When this reaction is performed in a closed system reaches an equilibrium state A(g) ⇄ B(g). The following picture shows a representative sample of this system.
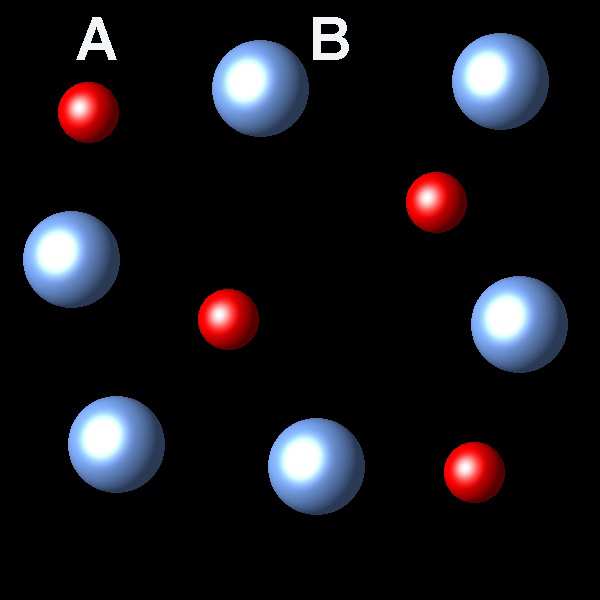 |
If the temperature of the system increases at constant pressure Which of the following representations best describes the situation of the system once reaches equilibrium again?
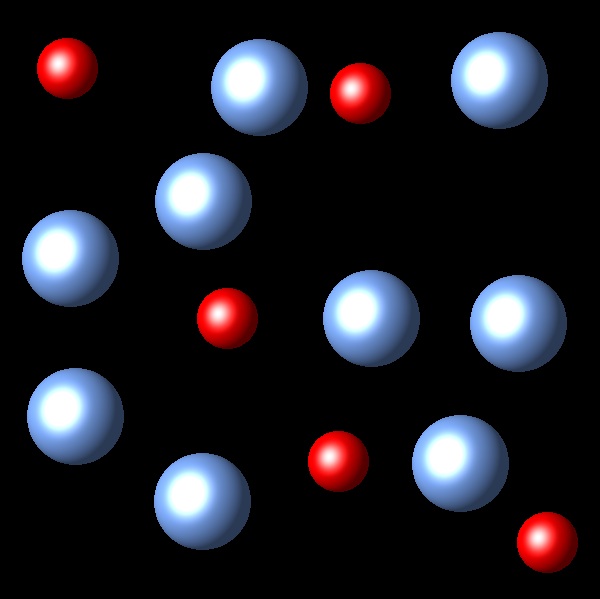 |
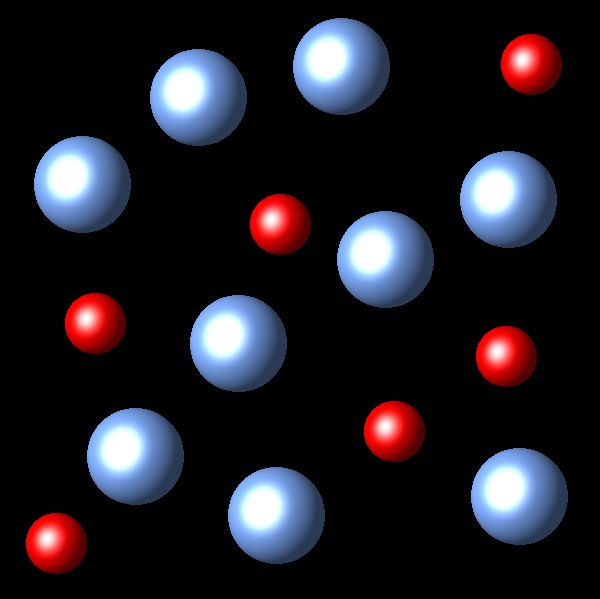 |
 |
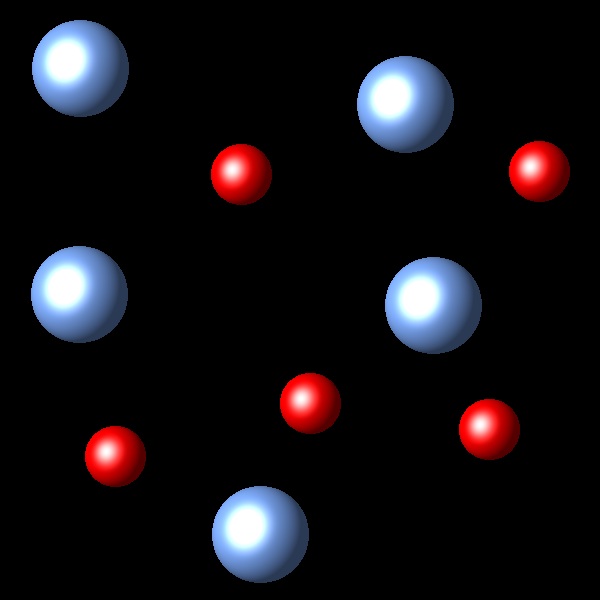 |
Volume changes are not shown in the figures.