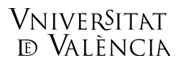|
|
Protein-protein interactions, both between soluble proteins as membrane level, are a key mechanism for cellular regulation. The characterization of these interactions, together with the identification of modulators, offers new opportunities to define therapeutic targets of interest. This project intends to use modulators of protein-protein interactions of biological relevance for the discovery of new drug candidates. The proposal focuses on two fundamental biological processes, programmed cell death (apoptosis) and integrin-mediated interactions responsible for the formation of platelet aggregates causing thrombosis and angiogenesis. The research project aims to use chemical biology techniques to modify / regulate cell signaling associated with these processes. | ||||||||
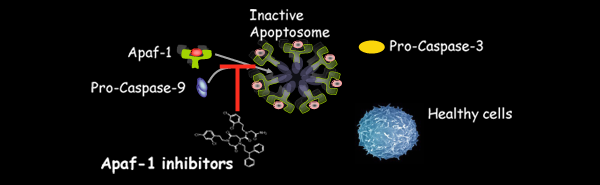
Membrane Proteins Group Biological membranes provide unique environments for biomolecular function. Biomembranes also define the cellular architecture and provide the foundation for cellular identity. The study of biomembranes is a major focus in contemporary life science research with important implications for essentially all fields of basic and applied biomolecular science. Membrane proteins are involved in an impressive range of biological functions (e.g., signalling, energy transduction, small molecule and ion transport, cell motility, cell-cell interactions, nerve conduction) and are targets for a majority of the pharmaceutical drugs currently on the market. About a quarter of cellular proteins are found embedded in lipid bilayers, where they perform critical functions. Yet, much of their structural and functional properties are still waiting to be unravelled. Peptide and Protein Chemistry LaboratoryApoptosis is executed by highly regulated cellular pathways and is crucial for the removal of cells with damaged DNA or organelles, viral or bacterial infections as well as superfluous or ectopic cells. Two major signalling pathways have been described for apoptosis induction. The extrinsic pathway is initiated through external stress signals that induce the binding of plasma membrane proteins of the death receptor family. The intrinsic pathway can be initiated by a number of factors including growth factor withdrawal and genotoxic stress which, with the participation of members of the Bcl-2 family of proteins, lead to mitochondrial outer membrane permeabilization (MOMP). After that signalling cascades activate the caspase family of cysteine aspartyl proteases. These caspases are essential to the initiation and execution of the apoptotic process. Venomics and Structural Proteomics Laboratory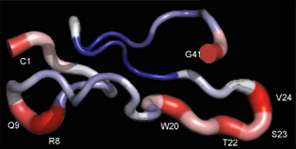
Snake envenoming constitutes a highly relevant public health issue in many tropical and subtropical countries, and has been recognised by the World Health Organization as a "neglected tropical disease". Adequate treatment of envenoming is critically dependent on the ability of antivenoms to neutralize the symptoms of systemic envenoming. A robust knowledge of the toxin composition and pathophysiological activities of venom proteomes is instrumental for the treatment of envenomed victims and for the selection of specimens for the generation of improved antidotes. A major research aim of our laboratoty is the development and application of proteomic tools (“venomics”, “antivenomics”, “venom phenotyping”) to study the natural history and composition of snake venoms and their interaction with antivenoms. Proteomic-guided identification of evolutionary and immunological trends among venoms may aid replacing the traditional geographic- and phylogenetic-driven hypotheses for antivenom production strategies by a more rationale approach based on venom proteome phenotyping and immunological profile similarities. Venoms comprise unique mixtures of deadly toxins tailored by Natural Selection in a prey-predator co-evolutionary arms race, and thus represent a sophisticated natural source of chemical and pharmacological novelty. Research on venoms (“venomics”) has emerged as a discovery science. In our laboratory we investigate structure-function correlations of the RTS-disintegrin jerdostatin, cloned from a venom gland cDNA library of the Chinese Jerdon's pitviper Protobothrops jerdonii, which selectively blocks the function of the α1β1 integrin both in vitro and in vivo. Jerdostatin may represent a valuable lead compound for the development of anti-angiogenic drugs. |
|||||||||