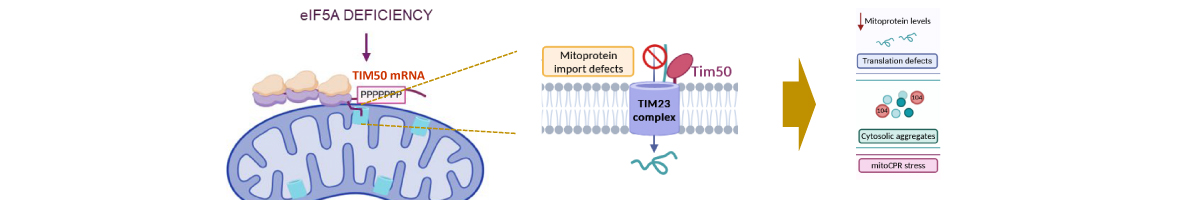
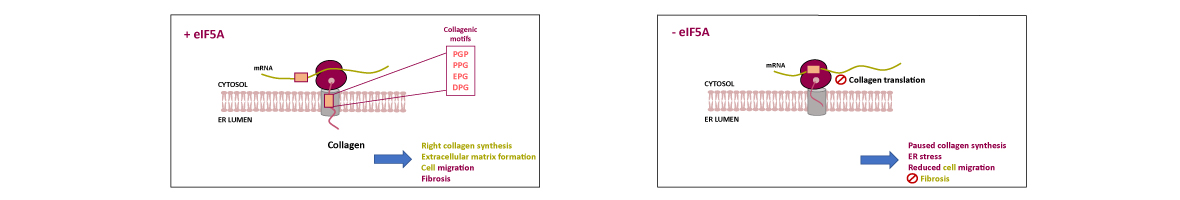
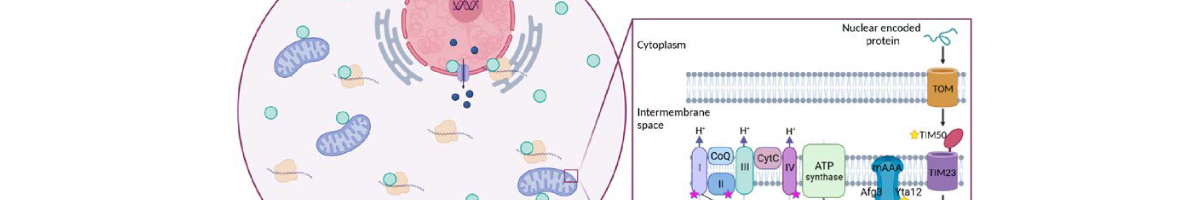
-
Clues to the origin of high external invertase activity in immobilized growing yeast: prolonged SUC2 transcription and less susceptibility of the enzyme to endogenous proteolysis
de Alteriis E, Alepuz PM, Estruch F, Parascandola P.
(1999). ArticleCanadian Journal of Microbiology (Can J Microbiol.). Num.Volume 45, Number 5 July 1999
Expression of the SUC2 gene encoding invertase was studied using free and gelatin-immobilized yeast cells to try to explain the high activity of this enzyme exhibited by immobilized cells when allowed to grow in a nutrient medium. The results indicated that at least two factors are probably responsible for the accumulation of invertase in immobilized cells. First, the expression of the SUC2 gene was maintained throughout growth in immobilized cells, whereas its expression was only transient in free cells. Second, invertase of immobilized cells was shown to be less susceptible to endogenous proteolytic attack than that of the corresponding free cells. These results have been interpreted,...
Expression of the SUC2 gene encoding invertase was studied using free and gelatin-immobilized yeast cells to try to explain the high activity of this enzyme exhibited by immobilized cells when allowed to grow in a nutrient medium. The results indicated that at least two factors are probably responsible for the accumulation of invertase in immobilized cells. First, the expression of the SUC2 gene was maintained throughout growth in immobilized cells, whereas its expression was only transient in free cells. Second, invertase of immobilized cells was shown to be less susceptible to endogenous proteolytic attack than that of the corresponding free cells. These results have been interpreted, respectively, in terms of diffusional limitations and changes in the pattern of invertase glycosylation due to growth of yeast in an immobilized state.Key words: immobilization, invertase, yeast, proteases.
Leer más Ocultar DOI: 10.1139/w99-024 -

Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p
Blondel M, Alepuz PM, Huang LS, Shaham S, Ammerer G, Peter M.
(1999). ArticleGenes & Development. Num.1999 Sep 1;13(17):2284-300
Far1p is a bifunctional protein that is required to arrest the cell cycle and to establish cell polarity during yeast mating. Far1p is localized predominantly in the nucleus but accumulates in the cytoplasm in cells exposed to pheromones. Here we show that Far1p functions in both subcellular compartments: nuclear Far1p is required to arrest the cell cycle, whereas cytoplasmic Far1p is involved in the establishment of cell polarity. The subcellular localization of Far1p is regulated by two mechanisms: (1) Far1p contains a functional bipartite nuclear localization signal (NLS), and (2) Far1p is exported from the nucleus by Msn5p/Ste21p, a member of the exportin family. Cells deleted for...
Far1p is a bifunctional protein that is required to arrest the cell cycle and to establish cell polarity during yeast mating. Far1p is localized predominantly in the nucleus but accumulates in the cytoplasm in cells exposed to pheromones. Here we show that Far1p functions in both subcellular compartments: nuclear Far1p is required to arrest the cell cycle, whereas cytoplasmic Far1p is involved in the establishment of cell polarity. The subcellular localization of Far1p is regulated by two mechanisms: (1) Far1p contains a functional bipartite nuclear localization signal (NLS), and (2) Far1p is exported from the nucleus by Msn5p/Ste21p, a member of the exportin family. Cells deleted for Msn5p/Ste21p failed to export Far1p in response to pheromones, whereas overexpression of Msn5p/Ste21p was sufficient to accumulate Far1p in the cytoplasm in the absence of pheromones. Msn5p/Ste21p was localized in the nucleus and interacted with Far1p in a manner dependent on GTP-bound Gsp1p. Two-hybrid analysis identified a small fragment within Far1p that is necessary and sufficient for binding to Msn5p/Ste21p, and is also required to export Far1p in vivo. Finally, similar to Δmsn5/ste21 strains, cells expressing a mutant Far1p, which can no longer be exported, exhibit a mating defect, but are able to arrest their cell cycle in response to pheromones. Taken together, our results suggest that nuclear export of Far1p by Msn5p/Ste21p coordinates the two separable functions of Far1p during mating.
Leer más Ocultar DOI: 10.1101/gad.13.17.2284 -
Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene
Alepuz PM, Cunningham KW, Estruch F.
(1997). ArticleMolecular Microbiology. Num.1997 Oct;26(1):91-8
In this report we show that the ENA1/PMR2A gene is under glucose repression. The SNF1 protein kinase, acting independently from the HOG and calcineurin pathways, is essential to release ENA1 from glucose repression. The transcriptional repressor Ssn6p negatively regulates ENA1 expression and, like other glucose repressible genes, this repression is mediated in part by Mig1p. Deletion of a fragment from the ENA1 promoter that includes two Mig1p consensus binding sites gives a high level of expression in glucose without added salt. We suggest that regulation of ENA1 by the SNF1 pathway could be part of a general mechanism through which yeast cells respond to carbon source starvation by...
In this report we show that the ENA1/PMR2A gene is under glucose repression. The SNF1 protein kinase, acting independently from the HOG and calcineurin pathways, is essential to release ENA1 from glucose repression. The transcriptional repressor Ssn6p negatively regulates ENA1 expression and, like other glucose repressible genes, this repression is mediated in part by Mig1p. Deletion of a fragment from the ENA1 promoter that includes two Mig1p consensus binding sites gives a high level of expression in glucose without added salt. We suggest that regulation of ENA1 by the SNF1 pathway could be part of a general mechanism through which yeast cells respond to carbon source starvation by activating protective systems against different types of stress.
Leer más Ocultar DOI: 10.1046/j.1365-2958.1997.5531917.x