The research group currently led by María Luisa Gil and Alberto Yáñez, called "Immunology of Fungal Infections," has focused its research over the past fifteen years on studying the host immune response to Candida albicans. The group has a multidisciplinary background, both in Microbiology and Immunology, making it ideally suited to study interactions between pathogenic fungi and immune system cells both in vitro and in vivo. Although the research is mainly basic, it has clear applied potential for the development of new immunotherapeutic approaches for the treatment of fungal infections.
C. albicans is an opportunistic pathogen that, depending on the host’s underlying conditions, can cause a range of infections, from superficial mucocutaneous candidiasis to severe invasive candidiasis. The frequency and severity of the latter have significantly increased in recent decades due to the growing population of immunocompromised or weakened individuals.
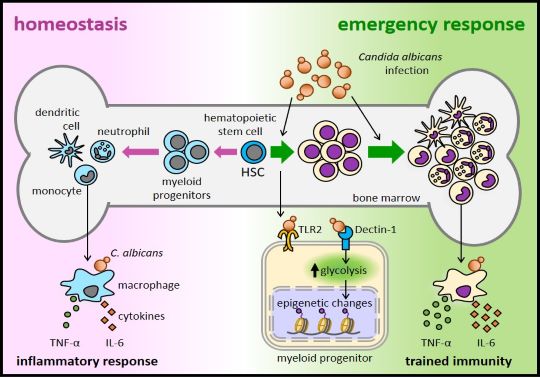
Resistance to candidiasis requires the coordinated action of innate and adaptive immune defenses. Mature innate immune cells use various PRRs (Pattern Recognition Receptors) to directly recognize MAMPs (Microbe-Associated Molecular Patterns), enabling them to detect a wide variety of pathogens with a limited number of receptors. The most important PRR families involved in the recognition of C. albicans are Toll-like receptors (TLRs) and C-type lectins (CLRs), such as Dectin-1. In this context, our group demonstrated that TLR2 is involved in recognizing C. albicans, both in yeast and hyphal forms, and in inducing cytokine and chemokine secretion through a pathway dependent on the adaptor molecule MyD88. This recognition is critical for protection against invasive candidiasis in a mouse infection model.
In 2006, it was reported that hematopoietic stem and progenitor cells (HSPCs)—which give rise to all immune system cells—express functional TLRs, and that TLR signaling in HSCs triggers cell cycle entry and differentiation toward the myeloid lineage. This discovery opened new perspectives on host-pathogen interactions, suggesting that these receptors could modulate hematopoiesis in response to microbes during infection. At that time, our group began studying the role of PRRs in C. albicans interactions with HSPCs and the consequences for infection resolution. In this line, we have shown that C. albicans induces proliferation and myeloid differentiation of HSPCs, both in vitro and in vivo. This response requires signaling through TLR2 and Dectin-1 and leads to the formation of functional macrophages capable of internalizing and killing yeasts and secreting inflammatory cytokines.
These results indicate that pathogens can be directly recognized by HSPCs through PRRs, promoting the replenishment capacity of the innate immune system during infection. Therefore, these receptors may be at least partly responsible for the emergency myelopoiesis that occurs in most infections, including invasive candidiasis (see Fig. 1).
In parallel with studies on innate immune memory, our group set a new objective: to study the function of phagocytes formed after HSPCs encounter microbial ligands. Using in vitro and in vivo models, we have demonstrated that PRR stimulation in HSPCs affects the functional phenotype of the macrophages they later generate. Therefore, our results show that this new concept of "trained immunity" can be applied not only to mature myeloid cells but also to HSPCs, increasing the durability of innate immune memory over time (Fig. 1).
Based on these results, and those of other authors in the same field, HSPCs are currently assigned an active role in combating infection. The hypothesis we are currently working on is that HSPCs can directly detect microorganisms and contribute to protection against infection through various mechanisms, including their capacity to differentiate into myeloid cells with an enhanced phenotype capable of facing pathogens and initiating the immune response.
The results already obtained open up new perspectives, potentially of great interest at the intersection of Immunology, Microbiology, and Hematology. The existence of new mechanisms in host-pathogen interaction, and their consequences on the modulation of the immune response during infection, could represent new therapeutic targets to boost immune responses in severe infections. In addition, microorganism-driven modulation of hematopoiesis could unveil new strategies for treating diseases involving altered myeloid cell production, such as myeloid leukemias.
.png)






