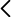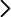Mechanism and performance of the hydrolytic chemical recycling of polylactide catalyzed by the protic ionic liquid 2-HEAA
- Authors: A. Cháfer, O. Gil-Castell, A. Björling, R. Ballesteros-Garrido, J.P.Cerisuelo-Ferriols, J.D.Badia (2024).
- Publication types: Article
- Publication Title (name of the book or magazine): Resources, Conservation and Recycling. No.Volume 210, November 2024, 107826
-
Abstract:
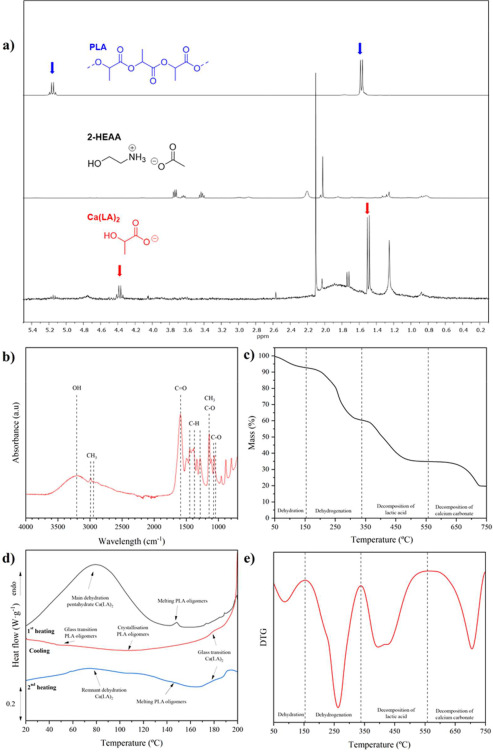
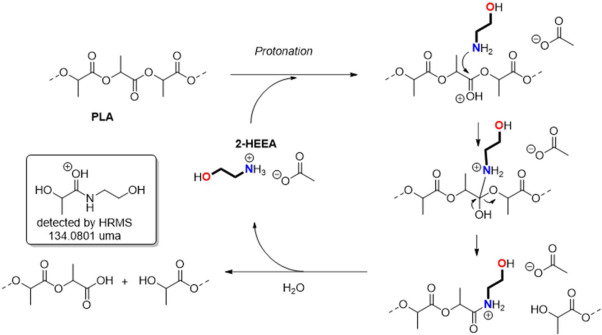
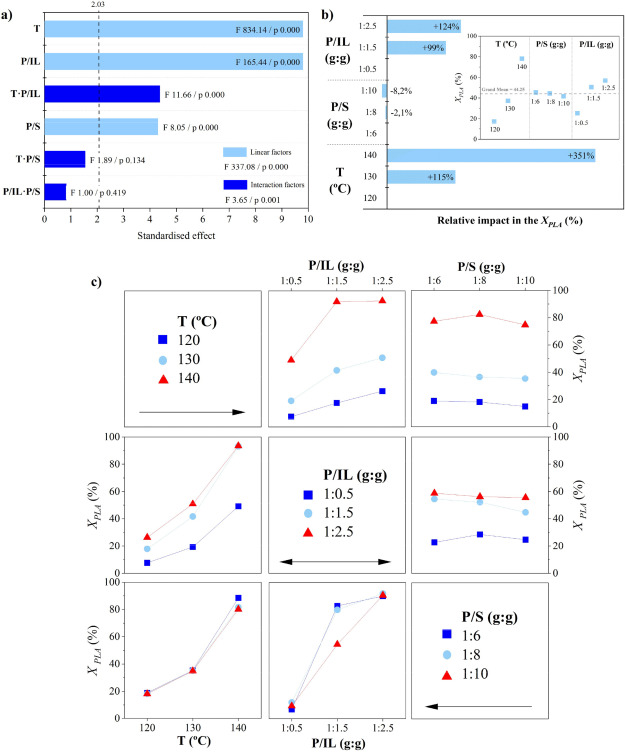
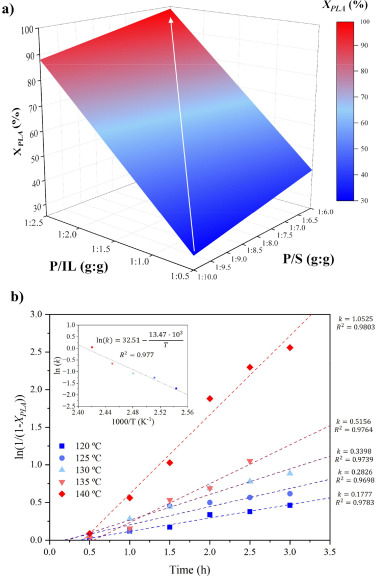
The hydrolytic chemical recycling of polylactide (PLA) aided by the protic ionic liquid 2-hydroxyethyl ammonium acetate (2-HEAA) as a homogeneous catalytic co-solvent was evaluated. In terms of maximization, temperature emerged as the most significant factor, being milder compared to that applied with other catalysts, resulting in potential energy savings. From a cost-efficiency perspective, employing a high solvent proportion was deemed unnecessary, thereby reducing reagent consumption. A higher proportion of ionic liquid leads to higher conversions, which could be trace-free recovered and reused in subsequent recycling steps. The best reaction triplet was a temperature of 140 °C, a mass ratio between PLA and water of 1:6, and a mass ratio between PLA and 2-HEAA of 1:2.5, enhancing the efficiency of PLA conversion under a waste-to-gate circular model in the chemical valorization industry. Thermal kinetics explained by a first-order model, show an apparent activation energy of 112.3 kJ·mol-1, which is lower compared to other ionic liquids.
Keywords: Chemical valorization; Hydrolysis; Polylactide (PLA); Protic ionic liquid (PIL); 2-hydroxyethyl ammonium acetate (2-HEAA); MechanismsKinetics
DOI: 10.1016/j.resconrec.2024.107826