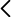- Universitat de València
- Research foundation of the Hospital Clínico de La Comunidad Valenciana (INCLIVA)
- Monteagudo Castro, Jose Carlos
- PDI-Catedratic/a d'Universitat
- José Francisco González Muñoz
- Beatriz Sánchez Sendra
Spitzoid cutaneous melanocytic tumours are a heterogeneous group of tumours that are often clinically and histopathologically ambiguous in terms of predicting their behaviour, many of them being atypical and nontypeable. Currently, the proposed diagnostic algorithms are insufficient for optimal clinical and therapeutic management. Therefore, there is a defensive tendency to overdiagnose malignancy, with the consequent implications in terms of time and physical, personal and economic resources (examination, check-ups, staging tests and treatments) as well as emotional strain. Thus, it is essential to incorporate new markers that allow for reliable and reproducible diagnostic assessment and predict the individualised metastatic potential of each patient.
Research staff from the Universitat de València, the Hospital Clínico Universitario de Valencia and from INCLIVA have developed a molecular signature of seen methylation sites of the human genome which, by means of an algorithm, makes it possible to reliably and repeatedly diagnose Spitzoid melanocytic tumours of uncertain malignant potential (MELTUMP) as benign or malignant and to predict the evolution of the patients’ metastic disease. MELTUMP is the group of cutaneous melanocytic tumours with the greatest diagnostic difficulty and clinical ambiguity.
Based on the seven methylation sites, an epigenetic diagnostic-health prognostic kit could be created for Spitzoid melanocytic tumours, capable of indicating the greater or lesser degree of malignancy of each tumour sample. This product could be of interest both to companies involved in clinical diagnostics and to pharmaceutical companies who, through further studies, could use these methylation sites as therapeutic targets..
The present invention has the following advantages:
- Risk-based follow-up planning for individual patients
- Avoidance of invasive surgical techniques such as sentinel lymph node biopsy or regional lymphadenectomy
- Optimisation of the health system
- Reduction of medical waiting lists
- Patent granted
Blasco Ibáñez Campus
C/ Amadeu de Savoia, 4
46010 València (València)