In 1913, the Danish physicist Niels Bohr published three articles in which proposed the first quantum model of the atom. The model gave a theoretical interpretation of the phenomenon of spectroscopy and allowed to interpret quantitatively the main features of the spectrum of the hydrogen atom. Bohr's atomic model was the first attempt of quantitative description of the structure of matter.
In 1913, the Danish physicist Niels Bohr published three articles in which he proposed the first quantum model of the atom [1,2,3]. The model gave a theoretical interpretation of the phenomenon of spectroscopy and allowed to interpret quantitatively the main features of the spectrum of the hydrogen atom.
Bohr's atomic model was the first attempt of quantitative description of the structure of matter and was the basis of the first descriptions of the chemical bond. It was also the starting point for the development of Erwin Schrödinger’s wave mechanics and the Werner Heisenberg’s matrix mechanics (who later became his disciple), the final models to interpret the structure of atoms. Niels Bohr received the Nobel Prize in Physics in 1922 "for his services in the investigation of the structure of atoms and of the radiation emanating from them". [4]
In the late nineteenth century, scientists believed that the physics building was virtually complete. Michelson, in 1899 (just a year before the discovery of Planck) had stated:
"The more important fundamental laws and facts of physical science have all been discovered; they are so firmly established that the possibility of being replaced, as a result of new discoveries, is remote. Our future discoveries only seek to obtain the sixth decimal figure."
But, only a year after, the quantum revolution began. In 1900, Max Planck proposed a theoretical interpretation of the distribution of the energy density of black body radiation [5] which can be considered the founding milestone of quantum theory, which necessarily implied that the energy exchange between the black body and radiation could not be done continuously but in the form of discrete packets known since then as "quanta" or quanta of energy.
In 1905, Albert Einstein presented a quite revolutionary and heterodox interpretation of the photoelectric effect, which included the need to extend the concept of Planck's quantum nature of the radiation. [6]
Einstein's hypothesis is based on:
"... the observations associated with black body radiation, fluorescence, cathode ray production by ultraviolet light and related phenomena, all connected with the emission or transformation of light, are more easily understood if one assumes that the light energy is distributed spatially in a discontinuous way... "
contradicting established ideas from Maxwell's equations on the wave nature (such as electromagnetic waves) and, therefore, continuous, of the radiation.
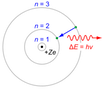
To resolve the interaction of radiation with matter, known experimentally by the phenomenon of atomic spectra, Bohr refined the Rutherford planetary model of the atom introducing the concept of Planck-Einstein energy quanta. The Bohr model is based on three postulates:
1.Electrons describe circular orbits around the atom nucleus without radiating energy
2.For electrons in atoms are only allowed those orbits whose radius meets the angular momentum of the electron is an integer multiple of h/2π
3.The electron only emits or absorbs energy in jumps from one allowed orbit to another. In this change emits or absorbs a photon whose energy is the energy difference between the two levels.
One of the main merits of the Bohr model is that justifies the mathematical relationships between different groups of spectral lines, which had been found empirically by Balmer and Rydberg. Although Bohr had attempted a general theory for the constitution of all atoms and molecules, in practice only explained the hydrogen atom. However, experiments and Hertz Frank (1914) and Stern and Gerlach (1922) respectively showed the existence of the stationary states postulated by Bohr and the spatial quantization.
The atomic model is not the only contribution of Niels Bohr to quantum mechanics. He contributed decisively to the formulation of the so-called "Copenhagen interpretation" of quantum mechanics, considered to be the orthodox interpretation and has been largely confirmed by all subsequent experiments. It is famous his controversy with Einstein on the consideration of quantum mechanics as a complete physical theory. [7,8]
From the beginning, the interpretation of the underlying theory in quantum mechanics divided physicists into two schools holding a lively debate. On the one hand, proponents of the "Copenhagen Interpretation", led by Bohr, among whom were Born, Heisenberg, Jordan, Pauli and Dirac. On the other hand, the "continuous Knights", commanded by Einstein and including among them some more of the fathers of quantum mechanics, such as Schrödinger, de Broglie, Planck, von Laue and Landé.
The heart of the matter lies, in the words of Einstein, if quantum mechanics itself is a "complete theory". In any case, the controversy focused on several aspects of the interpretation of quantum mechanics, not on its ability to predict experimental data. Einstein, the most representative of the physicists contrary to the Copenhagen interpretation says:
"... This interpretation does not describe what actually happens between observations or independently of them. But something must happen, that we cannot doubt, that something does not need to be described by electrons or quanta or waves, but unless you describe it in some way, the goal of physics is incomplete. It is not acceptable that refers only to the act of observation. Physicist must postulate in his science that he is exploring a world which he has not done and that might be present, essentially identical, if we were not here. Therefore the Copenhagen interpretation does not offer a real understanding of atomic phenomena."
For the Copenhagen school, the uncertainty relations are a constraint associated to the measurement process that do not reflect the limitations of the measuring device, but is intrinsic to the measurement process itself, and, therefore, will not disappear with the progress of the measurement technique. They represent a fundamental limitation which is permanently applied, and therefore, is a principle of nature, because it is only accessible to the knowledge the interaction of the system with the viewer. As Heisenberg said, those opposed to the Copenhagen interpretation:
"...prefer to return to the idea of an objective real-world, whose smallest parts exist objectively, such as stones and trees exist, regardless of whether or not we are observing. This, however, is impossible, or at least not entirely possible, because of the nature of the atomic events. "
The behaviour of a quantum particle is inherently statistical, and leaves no room for consideration of the causes of such behaviour; this phenomenon is not analysable.
Therefore, as Bohr himself concludes:
"...quantum mechanics implies the need to definitively renounce to the classical ideal of causality"
References:
1.- Bohr N. "On the constitution of atoms and molecules" Philosophical Magazine 26 (1913) 1-25
2.- Bohr N. "On the constitution of atoms and molecules" Philosophical Magazine 26 (1913) 476-502
3.- Bohr N. "On the constitution of atoms and molecules" Philosophical Magazine 26 (1913) 857-875
4.- http://www.nobelprize.org/nobel_prizes/physics/laureates/1922/press.html
5.- Planck M. "Sobre la teoría de la ley de distribución de la energía del espectro normal" Verhandlungen der Deutschen Physikalischen Gesellschaft 2 (1900) 237-245
6.- Einstein A. "Un punto de vista heurístico acerca de la creación y transformación de la luz", Annalen der Physik 17 (1905) 132-148
7.- Bohr, N. "Can Quantum-Mechanical Description of Physical Reality be Considered Complete?" Physical Review 48 (1935) 696-702
8.- Einstein, A., Podolsky B. and Rosen, N. "Can Quantum-Mechanical Description of Physical Reality be Considered Complete?" Physical Review 47 (1935) 777-780
More information:
On the Bohr atomic model:
http://en.wikipedia.org/wiki/Bohr_model
On Niels Bohr:
http://en.wikipedia.org/wiki/Niels_Bohr