Recently, Rafael Garcia-Meseguer and Iñaki Tuñón, members of the Physical Chemistry Department have published with colleagues from the University Jaume I, an article in Nature Chemistry that has had great impact. It shows the importance of the environment in enzymatic catalysis.
The enzymatic reactions develop over an overwhelming complexity. That's why their catalytic power is in many cases unknown or poorly understood. The study of enzymatic environment allows us to thoroughly study the evolution of the enzyme during catalysis and thus reveal some of their mysteries.
Enzymes play a crucial role in almost all biological processes accelerating metabolic reactions in several orders of magnitude and allowing them to occur on biological acceptable timescales. There is, therefore, a broad interest in understanding the origin of the catalytic power.
The conformational changes are indispensable for the enzymes to reach their full catalytic power allowing, among other things, the entrance and release of the reactants and products respectively. Still the impact of these structural movements during the time when the reaction is carried out is a controversial issue.
Many research studies suggest that these changes in the protein structure may have dynamic effects that produce a significant error in the calculation of the rate constant of the reaction by the transition state theory.
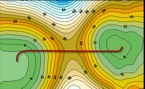
The reaction profile study based on both the environment and the solute is a powerful tool to elucidate the role of the structure and motions of the protein during the reaction step. The electrostatic potential is a property that can be used as way of controlling their evolving environment and thus the conformational changes, as it takes into account the influence of the environment along the reaction.
The application of this methodology as a model system is the haloalkane dehalogenase, leads to the conclusion that the catalytic efficiency of the enzyme should provide a favorable environment for the progress of the reaction. Although the flexibility of the protein is required to fully perform its catalytic effect, these movements do not produce significant dynamic corrections to the rate constant and therefore can be described as equilibrium fluctuations during the reaction.






