Molecular Biology and Biochemistry Department Researchers from the BioTecMed Institute and EGE-DtoP Group members (previously GFL) publish in Journal of Cell Biology (JCB) their study on eIF5A protein and its involvement in cancer and aging.
Universitat de València researchers Marina Barba-Aliaga, Vanessa Bernal and Paula Alepuz in collaboration with Dr. Brian Zid‘s group of California University (UCLA), published their study “eIF5A controls mitoprotein import by relieving ribosome stalling at TIM50 translocase mRNA” in JCB explaining how this protein keep mitochondrial breathing.
Mitochondria are the cell organelles in charge of energy production for cellular consumption. To this end, they need to continuously receive over 1000 different proteins that are codified by the nuclear genome and that will form the mitochondrial complexes in charge of metabolism routs such as the tricarboxylic acide cycle (TCA) or the mitochondrial breathing. eIF5A is a vital protein for every eukaryotic cell that has been preserved over evolution from yeast to humans and recent studies relate it to mitochondrial function.
EIF5A’s main molecular function is aiding cytosolic ribosomes during RNA messengers translation they codify for proteins containing aminoacids the have difficulties incorporating with the emerging polypeptid chain, like prolines. eIF5A has been associated with cancer, diabetes, viral infections and its drug-induced activation has been observed to increase several model organisms’ life-span and improve their immunological and cardiovascular systems as well as their cognitive skills during old age. Some of these beneficial effects are due to eIF5A’s improvement in protein synthetizing for mitochondria, which increases mitochondria energy production, although the molecular mechanism was unknown until now.
Final results
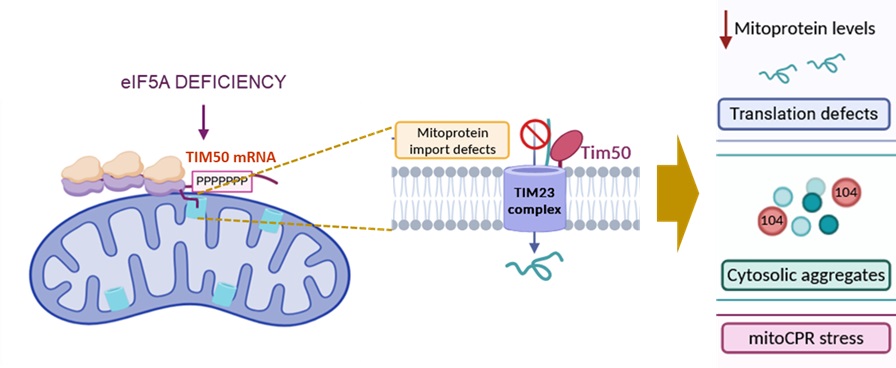
The results published on Journal of Cell Biology show how in yeast cells, eIF5A is necessary for the Codifying Messenger RNA translation of Tim50 mitochondria. The protein is vital and a part of the Tim23/Tim50 compound in the inner mitochondrial membrane, in charge of most of the mitochondrial proteins transportation from the cytosol to the mitochondria in both yeast and human cells. Tim50 Messenger RNA, like many other codifying RNA of mitochondrial proteins, is placed on the organelle’s surface so that its translation is connected to the transportation of the emerging protein.
Dr. Alepuz’ group found out that eIF5A must join the ribosomes translating Tim50 RNA to avoid clotting in a codifying region of consecutive prolines. If eIF5A are removed from yeast cells, the ribosomes are stuck and pile up in these RNA sequences on the mitochondrial surface, which then clots the protein transportation systems.
This clot lead to mitochondrial protein loss. The proteins are left on the cytosol, a stress response is sent and a reduction on codifying RNA translation of mitochondrial-destined proteins. The sole eIF5A manages the destination of most of mitochondrial proteins through controlling the synthesis of the Tim50 protein for the transportation system.
The results might show hos eIF5A promote the mitochondrial functioning during immune system activation or the decrease in eIF5A levels in old age may lead to a mitochondrial metabolism reduction and cellular deterioration.