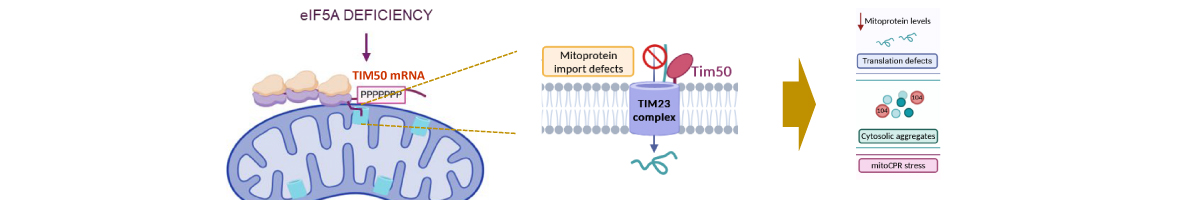
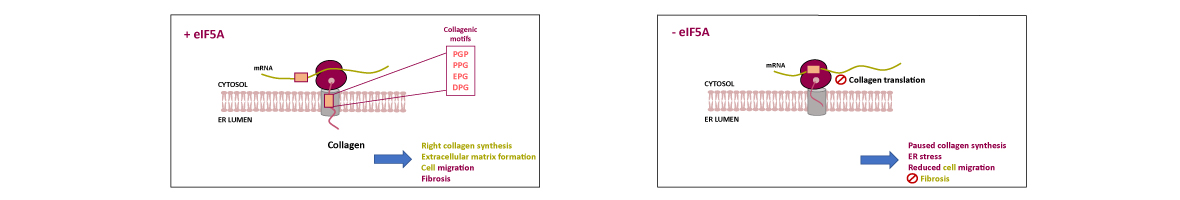
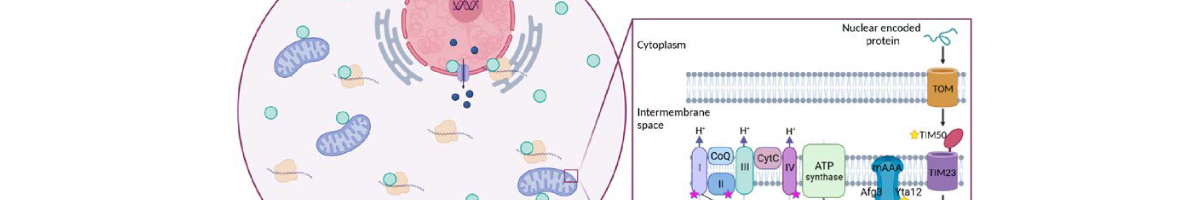
-

The bidirectional cytomegalovirus immediate/early promoter is regulated by Hog1 and the stress transcription factors Sko1 and Hot1 in yeast
Romero-Santacreu L, Orozco H, Garre E, Alepuz P.
(2010). ArticleMol Genet Genomics. No.2010 May;283(5):511-8
The work presented here intends to address the question of whether the immediate/early promoter of cytomegalovirus (CMV), which is widely used for expressing transgenes in eukaryotic cells, yields a constitutive expression of the transgenes under stress conditions in Saccharomyces cerevisiae cells. This information would also be relevant because in the tetracycline-regulated expression (tetO) system, which is one of the first choices for studying gene function from yeast to human cells, the CMV promoter controls the expression of the tetO transactivator. We found that the CMV promoter in yeast cells is bidirectionally induced by osmotic stress and in glycerol media. The mitogen-activated...
The work presented here intends to address the question of whether the immediate/early promoter of cytomegalovirus (CMV), which is widely used for expressing transgenes in eukaryotic cells, yields a constitutive expression of the transgenes under stress conditions in Saccharomyces cerevisiae cells. This information would also be relevant because in the tetracycline-regulated expression (tetO) system, which is one of the first choices for studying gene function from yeast to human cells, the CMV promoter controls the expression of the tetO transactivator. We found that the CMV promoter in yeast cells is bidirectionally induced by osmotic stress and in glycerol media. The mitogen-activated protein (MAP) kinase Hog1 controls CMV activation by osmotic stress through the ATF/CRE-related transcription factor Sko1 and the yeast osmostress factor Hot1. Our results indicate that the CMV and tetO expression systems respond to external signals and this should be considered before using these systems in yeast. Moreover, our results also suggest that CMV could be regulated by the intracellular glucose concentration in human cells.
Read more Hide DOI: 10.1007/s00438-010-0537-4 -
Repositorio Datos: Gene expression omnibus (GEO)
Romero-Santacreu L, Moreno J, Pérez-Ortín JE and Alepuz P
(2009). Recurs electrònicBiochim Biophys Acta-GRM. No.2015;1849(6):653-64
Acceso dataset: GSE13100. - Tipo datos: Genomic Run On (RNA expresión)
Datos citados en: RNA 2009; 15:1110-1120. Biochim Biophys Acta-GRM 2015;1849(6):653-64.
-

Specific and global regulation of mRNA stability during osmotic stress in Saccharomyces cerevisiae
Romero-Santacreu L, Moreno J, Pérez-Ortín JE, Alepuz P.
(2009). ArticleRNA. No.2009 Jun;15(6):1110-20
Hyperosmotic stress yields reprogramming of gene expression in Saccharomyces cerevisiae cells. Most of this response is orchestrated by Hog1, a stress-activated, mitogen-activated protein kinase (MAPK) homologous to human p38. We investigated, on a genomic scale, the contribution of changes in transcription rates and mRNA stabilities to the modulation of mRNA amounts during the response to osmotic stress in wild-type and hog1 mutant cells. Mild osmotic shock induces a broad mRNA destabilization; however, osmo-mRNAs are up-regulated by increasing both transcription rates and mRNA half-lives. In contrast, mild or severe osmotic stress in hog1 mutants, or severe osmotic stress in wild-type...
Hyperosmotic stress yields reprogramming of gene expression in Saccharomyces cerevisiae cells. Most of this response is orchestrated by Hog1, a stress-activated, mitogen-activated protein kinase (MAPK) homologous to human p38. We investigated, on a genomic scale, the contribution of changes in transcription rates and mRNA stabilities to the modulation of mRNA amounts during the response to osmotic stress in wild-type and hog1 mutant cells. Mild osmotic shock induces a broad mRNA destabilization; however, osmo-mRNAs are up-regulated by increasing both transcription rates and mRNA half-lives. In contrast, mild or severe osmotic stress in hog1 mutants, or severe osmotic stress in wild-type cells, yields global mRNA stabilization and sequestration of mRNAs into P-bodies. After adaptation, the absence of Hog1 affects the kinetics of P-bodies disassembly and the return of mRNAs to translation. Our results indicate that regulation of mRNA turnover contributes to coordinate gene expression upon osmotic stress, and that there are both specific and global controls of mRNA stability depending on the strength of the osmotic stress.
Read more Hide DOI: 10.1261/rna.1435709 -
Specific and global regulation of mRNA stability during osmotic stress in
Pérez-Ortín JE*, Alepuz PM, Moreno J.
(2007). ArticleTRENDS in Genetics. No.2007 May;23(5):250-7
As an adaptive response to new conditions, mRNA concentrations in eukaryotes are readjusted after any environmental change. Although mRNA concentrations can be modified by altering synthesis and/or degradation rates, the rapidity of the transition to a new concentration depends on the regulation of mRNA stability. There are several plausible transcriptional strategies following environmental change, reflecting different degrees of compromise between speed of response and cost of synthesis. The recent development of genomic techniques now enables researchers to determine simultaneously (either directly or indirectly) the transcription rates and mRNA half-lifes, together with mRNA...
As an adaptive response to new conditions, mRNA concentrations in eukaryotes are readjusted after any environmental change. Although mRNA concentrations can be modified by altering synthesis and/or degradation rates, the rapidity of the transition to a new concentration depends on the regulation of mRNA stability. There are several plausible transcriptional strategies following environmental change, reflecting different degrees of compromise between speed of response and cost of synthesis. The recent development of genomic techniques now enables researchers to determine simultaneously (either directly or indirectly) the transcription rates and mRNA half-lifes, together with mRNA concentrations, corresponding to all yeast genes. Such experiments could provide a new picture of the transcriptional response, by enabling us to characterize the kinetic strategies that are used by different genes under given environmental conditions.
Read more Hide*corresponding author.
DOI: 10.1016/j.tig.2007.03.006 -
The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes
De Nadal E, Zapater M, Alepuz PM, Sumoy L, Mas G, Posas F*.
(2006). ArticleNature. No.2004 Jan 22;427(6972):370-4
Regulation of gene expression by mitogen-activated protein kinases (MAPKs) is essential for proper cell adaptation to extracellular stimuli. Exposure of yeast cells to high osmolarity results in rapid activation of the MAPK Hog1, which coordinates the transcriptional programme required for cell survival on osmostress1. The mechanisms by which Hog1 and MAPKs in general regulate gene expression are not completely understood, although Hog1 can modify some transcription factors2. Here we propose that Hog1 induces gene expression by a mechanism that involves recruiting a specific histone deacetylase complex to the promoters of genes regulated by osmostress. Cells lacking the Rpd3–Sin3 histone...
Regulation of gene expression by mitogen-activated protein kinases (MAPKs) is essential for proper cell adaptation to extracellular stimuli. Exposure of yeast cells to high osmolarity results in rapid activation of the MAPK Hog1, which coordinates the transcriptional programme required for cell survival on osmostress1. The mechanisms by which Hog1 and MAPKs in general regulate gene expression are not completely understood, although Hog1 can modify some transcription factors2. Here we propose that Hog1 induces gene expression by a mechanism that involves recruiting a specific histone deacetylase complex to the promoters of genes regulated by osmostress. Cells lacking the Rpd3–Sin3 histone deacetylase complex are sensitive to high osmolarity and show compromised expression of osmostress genes. Hog1 interacts physically with Rpd3 in vivo and in vitro and, on stress, targets the deacetylase to specific osmostress-responsive genes. Binding of the Rpd3–Sin3 complex to specific promoters leads to histone deacetylation, entry of RNA polymerase II and induction of gene expression. Together, our data indicate that targeting of the Rpd3 histone deacetylase to osmoresponsive promoters by the MAPK Hog1 is required to induce gene expression on stress.
Read more Hide*corresponding author.
DOI: 10.1038/nature02258 -

A gene-specific requirement for FACT during transcription is related to the chromatin organization of the transcribed region
Jimeno-González S, Gómez-Herreros F, Alepuz PM, Chávez S*.
(2006). ArticleMolecular and Cellular Biology (MCB, Mol Cell Biol). No.2006 Dec;26(23):8710-21
The FACT complex stimulates transcription elongation on nucleosomal templates. In vivo experiments also involve FACT in the reassembly of nucleosomes traversed by RNA polymerase II. Since several features of chromatin organization vary throughout the genome, we wondered whether FACT is equally required for all genes. We show in this study that the in vivo depletion of Spt16, one of the subunits of Saccharomyces cerevisiae FACT, strongly affects transcription of three genes, GAL1, PHO5, and Kluyveromyces lactis LAC4, which exhibit positioned nucleosomes at their transcribed regions. In contrast, showing a random nucleosome structure, YAT1 and Escherichia coli lacZ are only mildly influenced...
The FACT complex stimulates transcription elongation on nucleosomal templates. In vivo experiments also involve FACT in the reassembly of nucleosomes traversed by RNA polymerase II. Since several features of chromatin organization vary throughout the genome, we wondered whether FACT is equally required for all genes. We show in this study that the in vivo depletion of Spt16, one of the subunits of Saccharomyces cerevisiae FACT, strongly affects transcription of three genes, GAL1, PHO5, and Kluyveromyces lactis LAC4, which exhibit positioned nucleosomes at their transcribed regions. In contrast, showing a random nucleosome structure, YAT1 and Escherichia coli lacZ are only mildly influenced by Spt16 depletion. We also show that the effect of Spt16 depletion on GAL1 expression is suppressed by a histone mutation and that the insertion of a GAL1 fragment, which allows the positioning of two nucleosomes, at the 5' end of YAT1 makes the resulting transcription unit sensitive to Spt16 depletion. These results indicate that FACT requirement for transcription depends on the chromatin organization of the 5' end of the transcribed region.
Read more HidePMID: 17000768 ; PMCID: PMC1636840
*corresponding author.
DOI: 10.1128/mcb.01129-06 -
Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II
Paula M. Alepuz, Eulàlia de Nadal, Meritxell Zapater, Gustav Ammerer*, Francesc Posas.
(2003). ArticleThe EMBO Journal. No.2003 May 15;22(10):2433-42
In budding yeast, the mitogen‐activated protein kinase (MAPK) Hog1 coordinates the transcriptional program required for cell survival upon osmostress. The Hot1 transcription factor acts downstream of the MAPK and regulates a subset of Hog1‐responsive genes. In response to high osmolarity, Hot1 targets Hog1 to specific osmostress‐responsive promoters. Here, we show that assembly of the general transcription machinery at Hot1‐dependent promoters depends on the presence of Hot1 and active Hog1 MAPK. Unexpectedly, recruitment of RNA polymerase (Pol) II complex to target promoters does not depend on the phosphorylation of the Hot1 activator by the MAPK. Hog1 interacts with the RNA Pol II and...
In budding yeast, the mitogen‐activated protein kinase (MAPK) Hog1 coordinates the transcriptional program required for cell survival upon osmostress. The Hot1 transcription factor acts downstream of the MAPK and regulates a subset of Hog1‐responsive genes. In response to high osmolarity, Hot1 targets Hog1 to specific osmostress‐responsive promoters. Here, we show that assembly of the general transcription machinery at Hot1‐dependent promoters depends on the presence of Hot1 and active Hog1 MAPK. Unexpectedly, recruitment of RNA polymerase (Pol) II complex to target promoters does not depend on the phosphorylation of the Hot1 activator by the MAPK. Hog1 interacts with the RNA Pol II and with general components of the transcription machinery. More over, when tethered to a promoter as a LexA fusion protein, Hog1 activates transcription in a stress‐ regulated manner. Thus, anchoring of active Hog1 to promoters by the Hot1 activator is essential for recruitment and activation of RNA Pol II. The mammalian p38 also interacts with the RNA Pol II, which might suggest a conserved mechanism for regulation of gene expression by SAPKs among eukaryotic cells.
Read more Hide*corresponding author.
DOI: 10.1093/emboj/cdg243 -
Dealing with osmostress through MAP kinase activation
Eulàlia de Nadal, Paula M Alepuz, and Francesc Posas*
(2002). ArticleThe EMBO Journal. No.Rep. 2002 Aug;3(8):735-40
In response to changes in the extracellular environment, cells coordinate intracellular activities to maximize their probability of survival and proliferation. Eukaryotic cells, from yeast to mammals, transduce diverse extracellular stimuli through the cell by multiple mitogen‐activated protein kinase (MAPK) cascades. Exposure of cells to increases in extracellular osmolarity results in rapid activation of a highly conserved family of MAPKs, known as stress‐activated MAPKs (SAPKs). Activation of SAPKs is essential for the induction of adaptive responses required for cell survival upon osmostress. Recent studies have begun to shed light on the broad effects of SAPK activation in the...
In response to changes in the extracellular environment, cells coordinate intracellular activities to maximize their probability of survival and proliferation. Eukaryotic cells, from yeast to mammals, transduce diverse extracellular stimuli through the cell by multiple mitogen‐activated protein kinase (MAPK) cascades. Exposure of cells to increases in extracellular osmolarity results in rapid activation of a highly conserved family of MAPKs, known as stress‐activated MAPKs (SAPKs). Activation of SAPKs is essential for the induction of adaptive responses required for cell survival upon osmostress. Recent studies have begun to shed light on the broad effects of SAPK activation in the modulation of several aspects of cell physiology, ranging from the control of gene expression to the regulation of cell division.
Read more HideREVIEW.
*corresponding author.
DOI: 10.1093/embo-reports/kvf158 -
Stress-induced map kinase Hog1 is part of transcription activation complexes
Alepuz PM, Jovanovic A, Reiser V, Ammerer G.*
(2001). ArticleMolecular Cell Research. No.2001 Apr;7(4):767-77
In response to hyperosmotic environments, most eukaryotic cells activate a specialized mitogen-activated protein (MAP) kinase pathway. In S. cerevisiae, the key protein kinase, Hog1, coordinates the transcriptional induction of a variety of genes devoted to osmoadaptation and general stress protection. Depending on the promoter context, Hog1 can function through a variety of structurally unrelated transcription factors. Using chromatin precipitation assays, we discovered that the kinase itself becomes intimately linked with promoter regions during stress responses. This interaction is dependent on the presence of stress-mediating transcriptional activators. In turn, Hog1 modulates promoter...
In response to hyperosmotic environments, most eukaryotic cells activate a specialized mitogen-activated protein (MAP) kinase pathway. In S. cerevisiae, the key protein kinase, Hog1, coordinates the transcriptional induction of a variety of genes devoted to osmoadaptation and general stress protection. Depending on the promoter context, Hog1 can function through a variety of structurally unrelated transcription factors. Using chromatin precipitation assays, we discovered that the kinase itself becomes intimately linked with promoter regions during stress responses. This interaction is dependent on the presence of stress-mediating transcriptional activators. In turn, Hog1 modulates promoter association of at least one of these factors. Additional findings highlight the possibility that Hog1 constitutes an integral part of the upstream activation complex, perhaps targeting not only the activator but also components of the general transcription machinery.
Read more HideReseña en STKE perspectives por el Dr. Chellappan, referente en el área (Sci STKE. 2001 Vol.93. HOG on the promoter: regulation of the osmotic stress response. DOI: 10.1126/stke.2001.93.pe1)
*corresponding author.
DOI: 10.1016/s1097-2765(01)00221-0 -

The Saccharomyces cerevisiae RanGTP-Binding Protein Msn5p Is Involved in Different Signal Transduction Pathways
Paula M Alepuz, Dina Matheos, Kyle W Cunningham, Francisco Estruch*
(1999). ArticleGenetics. No.Volume 153, Issue 3, 1 November 1999, Pages 1219–1231
In eukaryotes, control of transcription by extracellular signals involves the translocation to the nucleus of at least one component of the signal transduction pathway. Transport through the nuclear envelope requires the activity of an import or export receptor that interacts with the small GTPase Ran. We have cloned the MSN5 gene of the yeast Saccharomyces cerevisiae that is postulated to encode one of these receptors. Msn5p belongs to a family of proteins with a conserved N-terminal sequence that acts as a RanGTP-binding domain. The results presented here provide genetic data supporting Msn5p involvement in several different signal transduction pathways. All of these pathways include...
In eukaryotes, control of transcription by extracellular signals involves the translocation to the nucleus of at least one component of the signal transduction pathway. Transport through the nuclear envelope requires the activity of an import or export receptor that interacts with the small GTPase Ran. We have cloned the MSN5 gene of the yeast Saccharomyces cerevisiae that is postulated to encode one of these receptors. Msn5p belongs to a family of proteins with a conserved N-terminal sequence that acts as a RanGTP-binding domain. The results presented here provide genetic data supporting Msn5p involvement in several different signal transduction pathways. All of these pathways include changes in gene expression, and regulated nucleocytoplasmic redistribution of a component in response to external conditions has already been described in some of them. We have cloned MSN5 following two different strategies. Msn5p was constitutively localized in the nucleus. Phenotypic analysis of the msn5 mutant demonstrated that this protein participates in processes such as catabolite repression, calcium signaling, mating, and cell proliferation, as well as being involved in previously characterized phosphate utilization. Therefore, Msn5p could be a receptor for several proteins involved in different signaling pathways.
Read more Hide*corresponding author.
DOI: 10.1093/genetics/153.3.1219