-
Use of HSA(LR) female mice as a model for the study of myotonic dystrophy type I.
Carrascosa-Sàez M, Colom-Rodrigo A, González-Martínez I, Pérez-Gómez R, García-Rey A, Piqueras-Losilla D, Ballestar A, Llamusí B, Cerro-Herreros E, Artero R - 2025 - Lab Anim (NY)
(2025).ArticleDOI: 10.1038/s41684-025-01506-7 -
Erratum: Immortalized human myotonic dystrophy type 1 muscle cell lines to address patient heterogeneity
Núñez-Manchón J, Capó J, Martínez-Piñeiro A, Juanola E, Pesovic J, Mosqueira-Martín L, González-Imaz K, Maestre-Mora P, Odria R, Cerro-Herreros E, Naldaiz-Gastesi N, López de Munain A, Artero R, Savic-Pavicevic D, Vallejo-Illarramendi A, Mamchaoui K, Bigot A, Mouly V, Suelves M, Nogales-Gadea G - 2024 - iScience
(2024).Article[This corrects the article DOI: 10.1016/j.isci.2024.109930.].
DOI: 10.1016/j.isci.2024.111499 -
A Novel Class of FKBP12 Ligands Rescues Premature Aging Phenotypes Associated with Myotonic Dystrophy Type 1
García-Puga M, Gerenu G, Bargiela A, Espinosa-Espinosa J, Mosqueira-Martín L, Sagartzazu-Aizpurua M, Aizpurua JM, Vallejo-Illarramendi A, Artero R, López de Munain A, Matheu A - 2024 - Cells
(2024).ArticleBackground: Myotonic dystrophy type 1 (DM1) is an autosomal dominant disorder clinically characterized by progressive muscular weakness and multisystem degeneration, which correlates with the size of CTG expansion and MBLN decrease. These changes induce a calcium and redox homeostasis imbalance in several models that recapitulate the features of premature tissue aging. In this study, we characterized the impact of a new family of FKBP12 ligands (generically named MPs or MP compounds) designed to stabilize FKBP12 binding to the ryanodine receptors and normalize calcium dysregulation under oxidative stress. Methods: Human primary fibroblasts from DM1 patients and control donors, treated with...
Background: Myotonic dystrophy type 1 (DM1) is an autosomal dominant disorder clinically characterized by progressive muscular weakness and multisystem degeneration, which correlates with the size of CTG expansion and MBLN decrease. These changes induce a calcium and redox homeostasis imbalance in several models that recapitulate the features of premature tissue aging. In this study, we characterized the impact of a new family of FKBP12 ligands (generically named MPs or MP compounds) designed to stabilize FKBP12 binding to the ryanodine receptors and normalize calcium dysregulation under oxidative stress. Methods: Human primary fibroblasts from DM1 patients and control donors, treated with MP compounds or not, were used for functional studies of cell viability, proliferation, and metabolism. The gene expression profile in treated cells was determined using RNA sequencing. The impact of MP compounds in vivo was evaluated in a Drosophila model of the disease using locomotor activity and longevity studies. Results: The treatment with different MP compounds reversed oxidative stress and impaired cell viability and proliferation, mitochondrial activity, and metabolic defects in DM1-derived primary fibroblasts. RNA sequencing analysis confirmed the restoration of molecular pathways related to calcium and redox homeostasis and additional pathways, including the cell cycle and metabolism. This analysis also revealed the rescue of alternative splicing events in DM1 fibroblasts treated with MP compounds. Importantly, treatment with MP compounds significantly extended the lifespan and improved the locomotor activity of a Drosophila model of the DM1 disease, and restored molecular defects characteristic of the disease in vivo. Conclusions: Our results revealed that MP compounds rescue multiple premature aging phenotypes described in DM1 models and decipher the benefits of this new family of compounds in the pre-clinical setting of DM1. Keywords: FKBP12/RyR interaction; extended longevity; multisystem recovery; myotonic dystrophy; oxidative stress.
Read moreHide DOI: 10.3390/cells13231939 -
Decoding Nucleotide Repeat Expansion Diseases: Novel Insights from Drosophila melanogaster Studies.
Atienzar-Aroca S, Kat M, López-Castel A - 2024 - Int J Mol Sci
(2024).ArticleDrosophila melanogaster usage has provided substantial insights into the pathogenesis of several nucleotide repeat expansion diseases (NREDs), a group of genetic diseases characterized by the abnormal expansion of DNA repeats. Leveraging the genetic simplicity and manipulability of Drosophila, researchers have successfully modeled close to 15 NREDs such as Huntington's disease (HD), several spinocerebellar ataxias (SCA), and myotonic dystrophies type 1 and 2 (DM1/DM2). These models have been instrumental in characterizing the principal associated molecular mechanisms: protein aggregation, RNA toxicity, and protein function loss, thus recapitulating key features of human disease. Used in...
Drosophila melanogaster usage has provided substantial insights into the pathogenesis of several nucleotide repeat expansion diseases (NREDs), a group of genetic diseases characterized by the abnormal expansion of DNA repeats. Leveraging the genetic simplicity and manipulability of Drosophila, researchers have successfully modeled close to 15 NREDs such as Huntington's disease (HD), several spinocerebellar ataxias (SCA), and myotonic dystrophies type 1 and 2 (DM1/DM2). These models have been instrumental in characterizing the principal associated molecular mechanisms: protein aggregation, RNA toxicity, and protein function loss, thus recapitulating key features of human disease. Used in chemical and genetic screenings, they also enable us to identify promising small molecules and genetic modifiers that mitigate the toxic effects of expanded repeats. This review summarizes the close to 150 studies performed in this area during the last seven years. The relevant highlights are the achievement of the first fly-based models for some NREDs, the incorporation of new technologies such as CRISPR for developing or evaluating transgenic flies containing repeat expanded motifs, and the evaluation of less understood toxic mechanisms in NREDs such as RAN translation. Overall, Drosophila melanogaster remains a powerful platform for research in NREDs. Keywords: Drosophila melanogaster; high-throughput screening; protein aggregation; protein loss-of-function; rare disease; repeat expansion; toxic RNA.
Read moreHide DOI: 10.3390/ijms252111794 -
AntimiR treatment corrects myotonic dystrophy primary cell defects across several CTG repeat expansions with a dual mechanism of action
Cerro-Herreros E, Núñez-Manchón J, Naldaiz-Gastesi N, Carrascosa-Sàez M, García-Rey A, Losilla DP, González-Martínez I, Espinosa-Espinosa J, Moreno K, Poyatos-García J, Vilchez JJ, de Munain AL, Suelves M, Nogales-Gadea G, Llamusí B, Artero R - 2024 - Sci Adv
(2024).ArticleThis study evaluated therapeutic antimiRs in primary myoblasts from patients with myotonic dystrophy type 1 (DM1). DM1 results from unstable CTG repeat expansions in the DMPK gene, leading to variable clinical manifestations by depleting muscleblind-like splicing regulator protein MBNL1. AntimiRs targeting natural repressors miR-23b and miR-218 boost MBNL1 expression but must be optimized for a better pharmacological profile in humans. In untreated cells, miR-23b and miR-218 were up-regulated, which correlated with CTG repeat size, supporting that active MBNL1 protein repression synergizes with the sequestration by CUG expansions in DMPK. AntimiR treatment improved RNA toxicity readouts and...
This study evaluated therapeutic antimiRs in primary myoblasts from patients with myotonic dystrophy type 1 (DM1). DM1 results from unstable CTG repeat expansions in the DMPK gene, leading to variable clinical manifestations by depleting muscleblind-like splicing regulator protein MBNL1. AntimiRs targeting natural repressors miR-23b and miR-218 boost MBNL1 expression but must be optimized for a better pharmacological profile in humans. In untreated cells, miR-23b and miR-218 were up-regulated, which correlated with CTG repeat size, supporting that active MBNL1 protein repression synergizes with the sequestration by CUG expansions in DMPK. AntimiR treatment improved RNA toxicity readouts and corrected regulated exon inclusions and myoblast defects such as fusion index and myotube area across CTG expansions. Unexpectedly, the treatment also reduced DMPK transcripts and ribonuclear foci. A leading antimiR reversed 68% of dysregulated genes. This study highlights the potential of antimiRs to treat various DM1 forms across a range of repeat sizes and genetic backgrounds by mitigating MBNL1 sequestration and enhancing protein synthesis.
Read moreHide DOI: 10.1126/sciadv.adn6525 -
Msi2 enhances muscle dysfunction in a myotonic dystrophy type 1 mouse model
Sabater-Arcis M, Moreno N, Sevilla T, Perez Alonso M, Bargiela A, Artero R - 2024 - Biomed J
(2024).ArticleBackground: Myotonic dystrophy type 1 (DM1) is a rare neuromuscular disease caused by a CTG repeat expansion in the 3' untranslated region of the DM1 protein kinase gene. Characteristic degenerative muscle symptoms include myotonia, atrophy, and weakness. We previously proposed an Musashi homolog 2 (MSI2)>miR-7>autophagy axis whereby MSI2 overexpression repressed miR-7 biogenesis that subsequently de-repressed muscle catabolism through excessive autophagy. Because the DM1 HSALR mouse model expressing expanded CUG repeats shows weak muscle-wasting phenotypes, we hypothesized that MSI2 overexpression was sufficient to promote muscle dysfunction in vivo. Methods: By means of recombinant AAV...
Background: Myotonic dystrophy type 1 (DM1) is a rare neuromuscular disease caused by a CTG repeat expansion in the 3' untranslated region of the DM1 protein kinase gene. Characteristic degenerative muscle symptoms include myotonia, atrophy, and weakness. We previously proposed an Musashi homolog 2 (MSI2)>miR-7>autophagy axis whereby MSI2 overexpression repressed miR-7 biogenesis that subsequently de-repressed muscle catabolism through excessive autophagy. Because the DM1 HSALR mouse model expressing expanded CUG repeats shows weak muscle-wasting phenotypes, we hypothesized that MSI2 overexpression was sufficient to promote muscle dysfunction in vivo. Methods: By means of recombinant AAV murine MSI2 was overexpressed in neonates HSALR mice skeletal muscle to induce DM1-like phenotypes. Results: Sustained overexpression of the murine MSI2 protein in HSALR neonates induced autophagic flux and expression of critical autophagy proteins, increased central nuclei and reduced myofibers area, and weakened muscle strength. Importantly, these changes were independent of MBNL1, MBNL2, and Celf1 protein levels, which remained unchanged upon Msi2 overexpression. Conclusions: Globally, molecular, histological, and functional data from these experiments in the HSALR mouse model confirms the pathological role of MSI2 expression levels as an atrophy-associated component that impacts the characteristic muscle dysfunction symptoms in DM1 patients. Keywords: Adeno-associated virus; HSA(LR); Msi2; Muscle atrophy; Myotonic dystrophy. © 2023 The Authors. Published by Elsevier B.V. on behalf of Chang Gung University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Read moreHide DOI: 10.1016/j.bj.2023.100667 -
Therapeutic potential of oleic acid supplementation in myotonic dystrophy muscle cell models
Moreno N, Sabater-Arcis M, Sevilla T, Alonso MP, Ohana J, Bargiela A, Artero R - 2024 - Biol Res
(2024).ArticleBackground: We recently reported that upregulation of Musashi 2 (MSI2) protein in the rare neuromuscular disease myotonic dystrophy type 1 contributes to the hyperactivation of the muscle catabolic processes autophagy and UPS through a reduction in miR-7 levels. Because oleic acid (OA) is a known allosteric regulator of MSI2 activity in the biogenesis of miR-7, here we sought to evaluate endogenous levels of this fatty acid and its therapeutic potential in rescuing cell differentiation phenotypes in vitro. In this work, four muscle cell lines derived from DM1 patients were treated with OA for 24 h, and autophagy and muscle differentiation parameters were analyzed. Results: We demonstrate...
Background: We recently reported that upregulation of Musashi 2 (MSI2) protein in the rare neuromuscular disease myotonic dystrophy type 1 contributes to the hyperactivation of the muscle catabolic processes autophagy and UPS through a reduction in miR-7 levels. Because oleic acid (OA) is a known allosteric regulator of MSI2 activity in the biogenesis of miR-7, here we sought to evaluate endogenous levels of this fatty acid and its therapeutic potential in rescuing cell differentiation phenotypes in vitro. In this work, four muscle cell lines derived from DM1 patients were treated with OA for 24 h, and autophagy and muscle differentiation parameters were analyzed. Results: We demonstrate a reduction of OA levels in different cell models of the disease. OA supplementation rescued disease-related phenotypes such as fusion index, myotube diameter, and repressed autophagy. This involved inhibiting MSI2 regulation of direct molecular target miR-7 since OA isoschizomer, elaidic acid (EA) could not cause the same rescues. Reduction of OA levels seems to stem from impaired biogenesis since levels of the enzyme stearoyl-CoA desaturase 1 (SCD1), responsible for converting stearic acid to oleic acid, are decreased in DM1 and correlate with OA amounts. Conclusions: For the first time in DM1, we describe a fatty acid metabolism impairment that originated, at least in part, from a decrease in SCD1. Because OA allosterically inhibits MSI2 binding to molecular targets, reduced OA levels synergize with the overexpression of MSI2 and contribute to the MSI2 > miR-7 > autophagy axis that we proposed to explain the muscle atrophy phenotype. Keywords: Fatty acid; MSI2; Myoblast differentiation; Oleic acid; SCD1; miR-7.
Read moreHide DOI: 10.1186/s40659-024-00496-z -
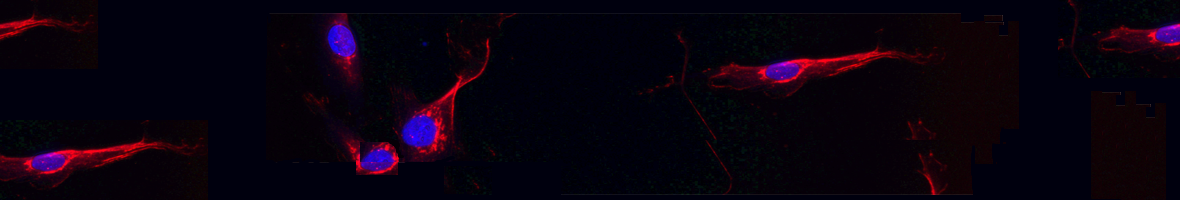
Immortalized human myotonic dystrophy type 1 muscle cell lines to address patient heterogeneity
Núñez-Manchón J, Capó J, Martínez-Piñeiro A, Juanola E, Pesovic J, Mosqueira-Martín L, González-Imaz K, Maestre-Mora P, Odria R, Cerro-Herreros E, Naldaiz-Gastesi N, López de Munain A, Artero R, Savic-Pavicevic D, Vallejo-Illarramendi A, Mamchaoui K, Bigot A, Mouly V, Suelves M, Nogales-Gadea G - 2024 - iScience
(2024).ArticleHistorically, cellular models have been used as a tool to study myotonic dystrophy type 1 (DM1) and the validation of therapies in said pathology. However, there is a need for in vitro models that represent the clinical heterogeneity observed in patients with DM1 that is lacking in classical models. In this study, we immortalized three DM1 muscle lines derived from patients with different DM1 subtypes and clinical backgrounds and characterized them at the genetic, epigenetic, and molecular levels. All three cell lines display DM1 hallmarks, such as the accumulation of RNA foci, MBNL1 sequestration, splicing alterations, and reduced fusion. In addition, alterations in early myogenic markers,...
Historically, cellular models have been used as a tool to study myotonic dystrophy type 1 (DM1) and the validation of therapies in said pathology. However, there is a need for in vitro models that represent the clinical heterogeneity observed in patients with DM1 that is lacking in classical models. In this study, we immortalized three DM1 muscle lines derived from patients with different DM1 subtypes and clinical backgrounds and characterized them at the genetic, epigenetic, and molecular levels. All three cell lines display DM1 hallmarks, such as the accumulation of RNA foci, MBNL1 sequestration, splicing alterations, and reduced fusion. In addition, alterations in early myogenic markers, myotube diameter and CTCF1 DNA methylation were also found in DM1 cells. Notably, the new lines show a high level of heterogeneity in both the size of the CTG expansion and the aforementioned molecular alterations. Importantly, these immortalized cells also responded to previously tested therapeutics. Altogether, our results show that these three human DM1 cellular models are suitable to study the pathophysiological heterogeneity of DM1 and to test future therapeutic options. Keywords: Biological sciences; Cell biology; Epigenetics; Health sciences; Molecular biology.
Read moreHide DOI: 10.1016/j.isci.2024.109930 -
Taurine activates the AKT-mTOR axis to restore muscle mass and contractile strength in human 3D in vitro models of steroid myopathy
Mughal S, Sabater-Arcis M, Artero R, Ramón-Azcón J, Fernández-Costa JM - 2024 - Dis Model Mech
(2024).ArticleSteroid myopathy is a clinically challenging condition exacerbated by prolonged corticosteroid use or adrenal tumors. In this study, we engineered a functional three-dimensional (3D) in vitro skeletal muscle model to investigate steroid myopathy. By subjecting our bioengineered muscle tissues to dexamethasone treatment, we reproduced the molecular and functional aspects of this disease. Dexamethasone caused a substantial reduction in muscle force, myotube diameter and induced fatigue. We observed nuclear translocation of the glucocorticoid receptor (GCR) and activation of the ubiquitin-proteasome system within our model, suggesting their coordinated role in muscle atrophy. We then examined...
Steroid myopathy is a clinically challenging condition exacerbated by prolonged corticosteroid use or adrenal tumors. In this study, we engineered a functional three-dimensional (3D) in vitro skeletal muscle model to investigate steroid myopathy. By subjecting our bioengineered muscle tissues to dexamethasone treatment, we reproduced the molecular and functional aspects of this disease. Dexamethasone caused a substantial reduction in muscle force, myotube diameter and induced fatigue. We observed nuclear translocation of the glucocorticoid receptor (GCR) and activation of the ubiquitin-proteasome system within our model, suggesting their coordinated role in muscle atrophy. We then examined the therapeutic potential of taurine in our 3D model for steroid myopathy. Our findings revealed an upregulation of phosphorylated AKT by taurine, effectively countering the hyperactivation of the ubiquitin-proteasomal pathway. Importantly, we demonstrate that discontinuing corticosteroid treatment was insufficient to restore muscle mass and function. Taurine treatment, when administered concurrently with corticosteroids, notably enhanced contractile strength and protein turnover by upregulating the AKT-mTOR axis. Our model not only identifies a promising therapeutic target, but also suggests combinatorial treatment that may benefit individuals undergoing corticosteroid treatment or those diagnosed with adrenal tumors. Keywords: 3D bioengineered skeletal muscle tissues; Corticosteroids; Steroid myopathy; Taurine.
Read moreHide DOI: 10.1242/dmm.050540 -
Antagonistic roles of canonical and Alternative-RPA in disease-associated tandem CAG repeat instability
Gall-Duncan T, Luo J, Jurkovic CM, Fischer LA, Fujita K, Deshmukh AL, Harding RJ, Tran S, Mehkary M, Li V, Leib DE, Chen R, Tanaka H, Mason AG, Lévesque D, Khan M, Razzaghi M, Prasolava T, Lanni S, Sato N, Caron MC, Panigrahi GB, Wang P, Lau R, Castel AL, Masson JY, Tippett L, Turner C, Spies M, La Spada AR, Campos EI, Curtis MA, Boisvert FM, Faull RLM, Davidson BL, Nakamori M, Okazawa H, Wold MS, Pearson CE - 2023 - Cell
(2023).ArticleExpansions of repeat DNA tracts cause >70 diseases, and ongoing expansions in brains exacerbate disease. During expansion mutations, single-stranded DNAs (ssDNAs) form slipped-DNAs. We find the ssDNA-binding complexes canonical replication protein A (RPA1, RPA2, and RPA3) and Alternative-RPA (RPA1, RPA3, and primate-specific RPA4) are upregulated in Huntington disease and spinocerebellar ataxia type 1 (SCA1) patient brains. Protein interactomes of RPA and Alt-RPA reveal unique and shared partners, including modifiers of CAG instability and disease presentation. RPA enhances in vitro melting, FAN1 excision, and repair of slipped-CAGs and protects against CAG expansions in human cells. RPA...
Expansions of repeat DNA tracts cause >70 diseases, and ongoing expansions in brains exacerbate disease. During expansion mutations, single-stranded DNAs (ssDNAs) form slipped-DNAs. We find the ssDNA-binding complexes canonical replication protein A (RPA1, RPA2, and RPA3) and Alternative-RPA (RPA1, RPA3, and primate-specific RPA4) are upregulated in Huntington disease and spinocerebellar ataxia type 1 (SCA1) patient brains. Protein interactomes of RPA and Alt-RPA reveal unique and shared partners, including modifiers of CAG instability and disease presentation. RPA enhances in vitro melting, FAN1 excision, and repair of slipped-CAGs and protects against CAG expansions in human cells. RPA overexpression in SCA1 mouse brains ablates expansions, coincident with decreased ATXN1 aggregation, reduced brain DNA damage, improved neuron morphology, and rescued motor phenotypes. In contrast, Alt-RPA inhibits melting, FAN1 excision, and repair of slipped-CAGs and promotes CAG expansions. These findings suggest a functional interplay between the two RPAs where Alt-RPA may antagonistically offset RPA's suppression of disease-associated repeat expansions, which may extend to other DNA processes.
Read moreHide DOI: 10.1016/j.cell.2023.09.008
.png)