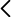- Universitat de València
- Università degli Studi di Torino
- Inserm Transfert
- Artero Allepuz, Ruben Dario
- PDI-Catedratic/a d'Universitat
- Piotr Konieczny
- Marina Marina Boido
- Giovanna Mendutti
- Camile Januel
- Cecile Martinat
Spinal Muscular Atrophy (SMA) is a rare neurodegenerative disease caused by the lack of the Survival of Motor Neuron protein (SMN). The prevalence of SMA is 4 in 100.000 people, and the clinical presentations range from severe motor dysfunction and infant mortality to minor defects with a normal lifespan. Three therapies have been approved to treat SMA; Spinraza™, Evrysdi™ and Zolgensma™. However, their extremely high costs (more than 200.000 euros per patient and year) can make them difficult to access for most patients and puts tremendous financial stress on the national health systems. Additionally, these therapies are not devoid of risks and limitations. Zolgensma™ is addressed only to the youngest and quickly diagnosed children. Spinraza™ requires highly invasive intrathecal administration, which isn´t always possible in all patients. The mechanism of action used by Evrysdi™ doesn´t allow fully restore the SMN levels to the healthy individual´s level. In addition, the long-term safety profile has not yet been confirmed, and it has been seen that some patients do not show improvement even after years of treatment, even worsening. Notably, in the case of adult patients, many of them do not even receive treatment, or their efficacy is very low. Thus, SMA patients are still in need of an effective therapy with a well-known long-term toxicological profile at a competitive price.
Reserachers of the Department of Genetics of Universitat de València, Università degli Studi di Torino and INSERM, have discovered an FDA-approved drug, haloperidol, which has the potential to become a therapy for SMA patients, or at least for some subtypes of patients. The researchers have used the approach of drug repurposing to search for new therapies for SMA. Haloperidol, a neuroleptic drug, has been tested on SMA type II mice model, widely used in preclinical studies. Results show increased SMN levels in the spinal cord and skeletal muscles, resulting in improved strength and dexterity of the treated mice. Half-life and weight gain of treated animals was also improved, and it was showed a neuroprotective and anti-inflammatory effect at the spinal cord level, which shows a SMN-dependent and independent effect of haloperidol. Additionally, rescue has been seen on phenotypes that other treatments do not improve, as is the case of neuromuscular junctions (NMJ), or motor neuron survival, where it shows a superior rescue. Notably, it not only causes changes in SMN splicing, but also increases its levels at the transcript level, also having effects outside the CNS. In contrast to currently licensed treatments, detailed knowledge is available on the pharmacological safety profile of the reassigned drug candidate, which shows an excellent safety and biodistribution profile in chronic use. The results obtained were consistent across three different laboratories, confirming reproducibility.
The main application of this invention is in the pharmaceutical sector, for the treatment of potentially all SMA types, including patients with mild symptoms for which access to costly drugs might be difficult, and their caregivers, through the national public or private healthcare systems.
The main advantages of this invention are:
- Excellent bio-distribution through animal tissues.
- Difussion through the blood-brain barrier.
- Reduced development timelines of the new drug candidate.
- Higher success rate on the regulatory process.
- Repurposed drugs can be tested in humans at safe doses to evaluate their efficacy.
- Comparatively lower cost to alternative treatments.
- Possibility of new administration routes of the treatment, adjusted to patient’s needs.
- Patent granted
Blasco Ibáñez Campus
C/ Amadeu de Savoia, 4
46010 València (València)