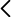In Alzheimer’s disease, the precursor cells of new neurons show an alteration in their cell cycle and a state of senescence that affects their ability to migrate, which could explain the loss of smell and other cognitive defects associated with this pathology. This is the main conclusion of a study led by the CIBER on Fragility and Healthy Aging (CIBERFES) and the University of Valencia (UV) that has just been published in the journal Molecular Neurobiology.
In the brain There are two areas in the brain where the processes of differentiation and formation of new neurons take place throughout life. Chief among these neurogenic niches is the subventricular zone in the forebrain, where complex interactions take place that induce neural stem cells and neural progenitors to proliferate, differentiate, and move to the appropriate position, in a process known as neuronal migration. In Alzheimer’s disease, the process of formation of new neurons is altered and there is a decrease in proliferation and neurogenesis that can promote cognitive defects.
In this new study, the research team focused on studying the capacity of the subventricular neurogenic niche cells in mice with Alzheimer’s disease, to understand the role of some proteins involved in this pathology and in the neural migration process. “We have been able to verify that this ability to generate new neurons is severely damaged and is a phenomenon that occurs before the typical lesions of the disease”, explains Ana Lloret, researcher at CIBERFES and the UV, and one of the coordinators of the study.
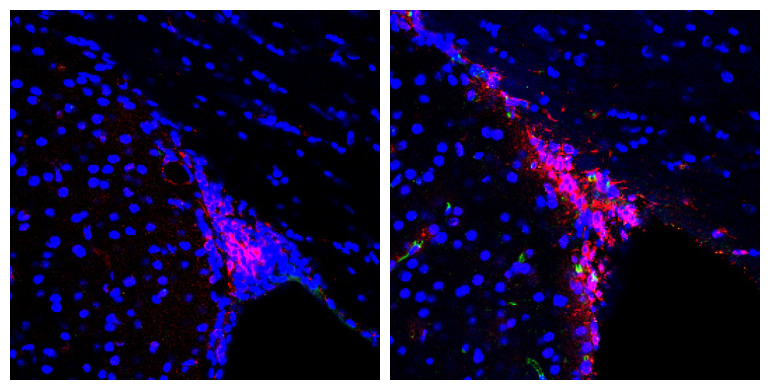
Specifically, in this work it was observed that mice with Alzheimer’s show a deficit in cell migration from this subventricular neural niche to its proper position, due to a state of senescence of these new neurons.
Cellular senescence is a process that begins as a response to stress and damage occurred in a cell and that causes it to present defects in its proliferation capacity. These senescent or aged cells stop their cell cycle although they do not die, and remain active by releasing harmful substances into their environment. Thus, they accumulate in body tissues and play an important role in the development of different pathologies, such as cancer or, in this case, Alzheimer.
Loss of smell in Alzheimer’s patients
In this work, this group of scientists confirmed that new neurons do form, but senescence makes them unable to migrate and they remain accumulated in the subventricular neural niche, which enlarges and grows abnormally in these mice.
“In humans, this neurogenic disability has not been described yet, but it could be the explanation for the loss of smell suffered by Alzheimer’s patients from the very early stages of the disease, since the neurogenic niche of the subventricular zone is the one that provides new neurons to the olfactory bulb”, explains José Viña, head of the CIBERFES group at the University of Valencia and another of the main authors of this work.
Likewise, they point out that this neural niche is the source of other types of brain cells, “so the same process of senescence and alteration of neural migration could explain other cognitive deficits associated with Alzheimer’s”.
“The results of this work could contribute to a new approach to Alzheimer’s based on senolytic compounds or proneurogenic factors”, point out the signatories.
About CIBERFES
The Network Biomedical Research Centre (CIBER) is a consortium dependent on the Carlos III Health Institute (Ministry of Science and Innovation) co-financed with FEDER funds. The CIBER for Fragility and Healthy Aging (CIBERFES) was created at the end of 2016 with the aim of understanding, evaluating and mitigating, as far as possible, frailty and its main consequence, disability, which many older people suffer from. The 20 research groups that form it, belonging to 18 consortium institutions, work on four main lines of research: study of the biological mechanisms of healthy aging and of those that lead to frailty and disability; use of cohorts to study the interaction between chronic disease, aging and functional impairment; preventive and therapeutic interventions in frailty and functional impairment; and care models.
Annex image: Confocal microscopy image where the migrating and senescent cells of the SVZ of a control mouse (left) and with Alzheimer’s disease (right) have been stained. As can be seen, there are many more migratory cells, which are also senescent, in the disease model.
Images: