Human diseases that originate from non-human reservoirs, zoonoses, constitute 75% of emerging infectious diseases and pose a significant threat to public health. In the particular case of Ebola, the 2014 epidemic in West Africa has been the largest registered ever, affecting tens of thousands individuals with mortality rates close to 75%. In addition, Ebola virus (EV) decimates the great ape population, thus posing a conservation hazard, represents a major threat worldwide through the importation of infections and its possible misuse as biological weapon, and has dramatic economic and humanitarian consequences.
The generation of mitotically stable alterations in gene expression due to epigenetic marks is a fast and relatively long-lasting manner for stablishing a genomic memory of past stress events. Environment-triggered deregulation of genetic factors associated with epigenetic machineries can also lead to phenotypic plasticity and stress mitigation. Therefore, host’s epigenetic machinery can pose an important but largely unmeasured selective pressure on pathogens. Plant viruses offer a convenient model for studying this kind of interactions. Firstly, a large-scale evolution experiment for checking how virus populations evolve and interact in plants with compromised or enhanced epigenetic pathways is proposed. Arabidopsis thaliana plants with mutations in key genes associated with active or repressive chromatin marks, including DNA methylation and histone modification, will be challenged against independent lineages of turnip mosaic potyvirus (TuMV).
Massively parallel or next-generation sequencing (NGS) has been a revolution in genetic studies, having important applications in research and in clinics. Some are the study of the genome, transcriptome, microbiome, methylome, genetic diagnosis and personalised medicine, such as pharmacogenetics and the detection of somatic tumour markers, studies of tumour mutations in circulating DNA, determination of the mutation rate for oncology immunotherapy studies. An important application is the study of specific regions of the genome for many clinical or research studies, mainly of one or several genes (NGS panel) due to the high costs of genome-wide studies.
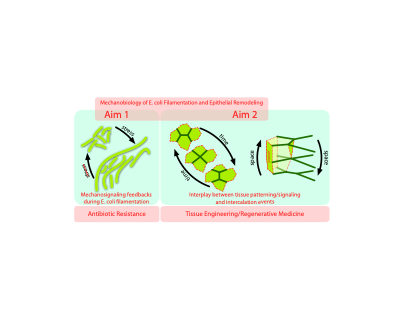
Antibiotic resistance and tissue engineering/regenerative medicine have been acknowledged as two of the grand research challenges of Biotechnology. While seemingly unconnected, we argue that providing quantitative answers to these questions requires a common approach from the viewpoint of Mechanobiology. On the one hand, the characteristic phenotypic readout of antibiotic resistance (and more in general of a stress response in bacteria) is filamentation.
The ability to resolve populations structure at high resolution and in high depth is key for understand viral evolution and pathogenesis. In this project, we are implementing an extreme fidelity next-generation based sequencing techniques to be able to address key questions in viral evolution and pathogenesis.
Contract of advice and technical support with EVERIS Company.
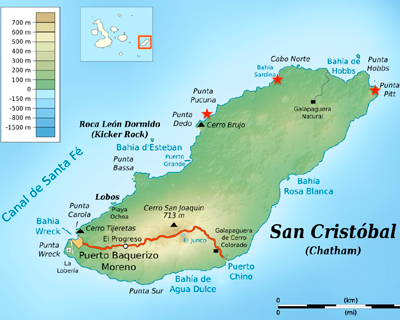
This project is coordinated by the Institute for Integrative Systems Biology I2SysBio) and the Universidad San Francisco de Quito. The project explores the presence of microorganisms with electricity generating potential of microbial communities present in the sediments of salt lagoons and salt flats of San Cristóbal Island in the Galapagos archipelago. Galapagos explores the production of electricity from environmental samples, characterizes the metagenomic content of microbial communities and the correlation of the presence of power species with environmental parameters such as pH, salinity, temperature and dissolved oxygen.
This project is coordinated by the Institute for Integrative Systems Biology I2SysBio) and the ICTS The Canfranc Underground Laboratory. The project studies the content and the spatial variability of the microbial communities present in limestone rocks samples from the Pyrenees at hundreds meters deep thanks to access thought the Somport tunnel, located in the Central Pyrenees and linking the Aragon (Spain) and Aspe (France) valleys. Gollum explores a little known extreme environment, characterized by few nutrients, various physicochemical substrates, low levels of any type of radiation and small temperature fluctuations. The presence of native DNA and the identification of a high archaea contents and its correlation with present metals are some of the most relevant results and open the possibility of multiple questions, starting by resolving whether genomic material identified corresponds to relic DNA or, on the contrary, to living microorganisms isolated from the outside since tens of millions of years.


 Risk Assessment of Ebola Outbreaks through Probabilistic Modeling of Chiroptera Zoonotic Dynamics and Socioeconomic Factors
Risk Assessment of Ebola Outbreaks through Probabilistic Modeling of Chiroptera Zoonotic Dynamics and Socioeconomic Factors
 EPICOVIR
EPICOVIR
 EOSAL_CLINIC
EOSAL_CLINIC
 Mechanobiology of E. coli Filamentation and Epithelial Remodeling
Mechanobiology of E. coli Filamentation and Epithelial Remodeling
 AICO
AICO
 Advising for the project implementation of development of an application of authorship of a sequence listing of nucleotides and amino acids in ST26 format
Advising for the project implementation of development of an application of authorship of a sequence listing of nucleotides and amino acids in ST26 format
 GALAPAGOS
GALAPAGOS
 GOLLUM
GOLLUM
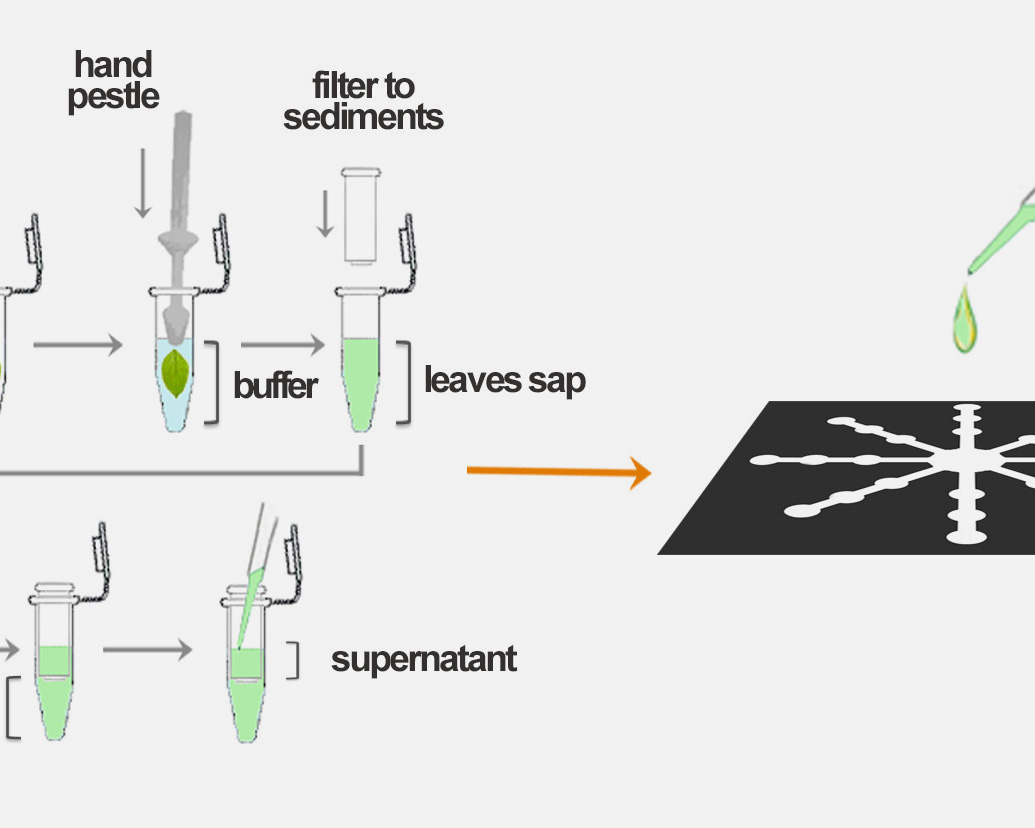 PLANT-PREDICTOR
PLANT-PREDICTOR
 EGReNMel
EGReNMel
 COV-CRISPIS
COV-CRISPIS
 NETFRUIT
NETFRUIT
 SYSY-RNA
SYSY-RNA
 RNAct
RNAct
 EvolDup2Inno
EvolDup2Inno
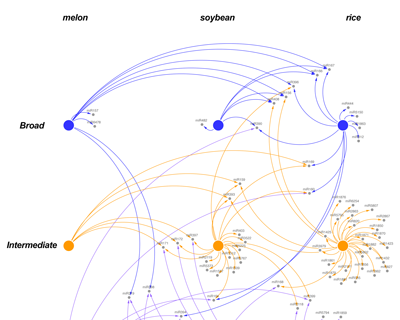 Functional validation of sncRNA networks which regulate the response to stress in melons
Functional validation of sncRNA networks which regulate the response to stress in melons
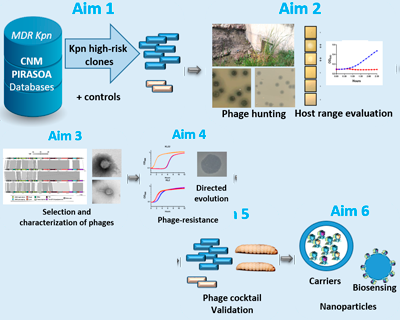 PHAGTHERKpn
PHAGTHERKpn
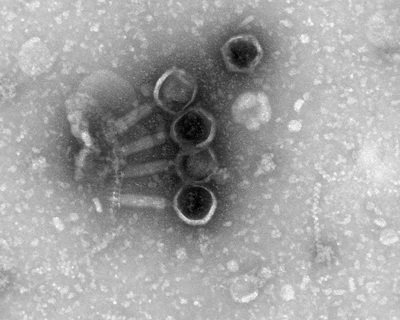 Superfago
Superfago
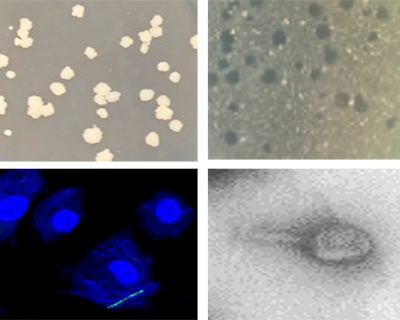 PhageMabs
PhageMabs
 PTI+: Transmission and contention
PTI+: Transmission and contention
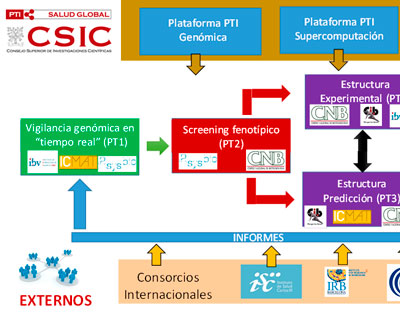 Genomic monitoring
Genomic monitoring
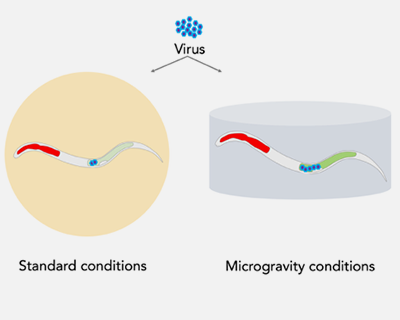 SpaceWorms
SpaceWorms
 Mutaciones
Mutaciones
 Antiviral platform
Antiviral platform
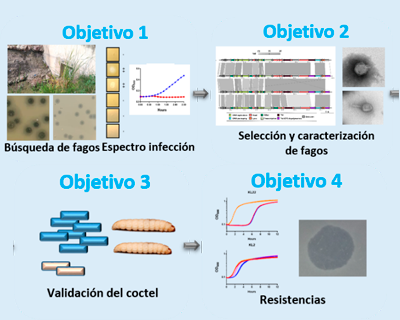 FagoterapiaKpn
FagoterapiaKpn
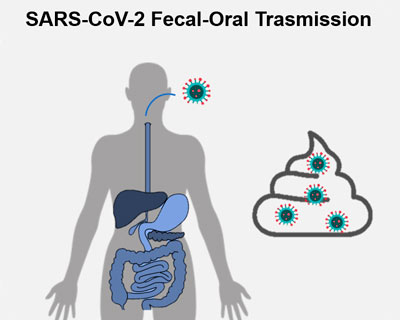 FecOrSARS
FecOrSARS
 COVID-19: anti-infectious and anti-inflammatory action of immunomodulatory parasite molecules in a safe-to-use synthetic format
COVID-19: anti-infectious and anti-inflammatory action of immunomodulatory parasite molecules in a safe-to-use synthetic format
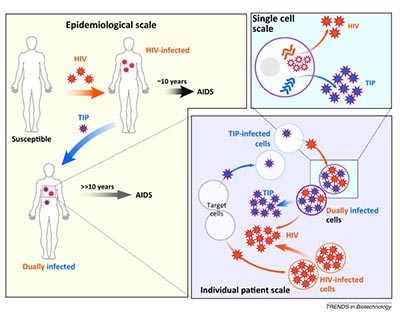 CoV2TIP
CoV2TIP
 CaeVirus
CaeVirus
 EnvironmentalCOVID
EnvironmentalCOVID
 BlockAce
BlockAce
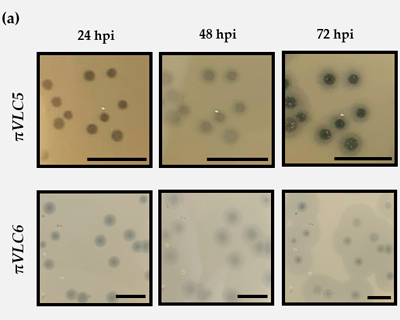 IsolationKpnPhages
IsolationKpnPhages
 SeqCOVID
SeqCOVID
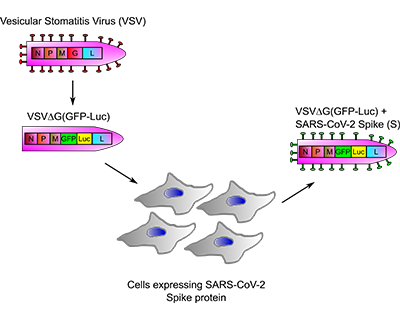 ANTICOR
ANTICOR
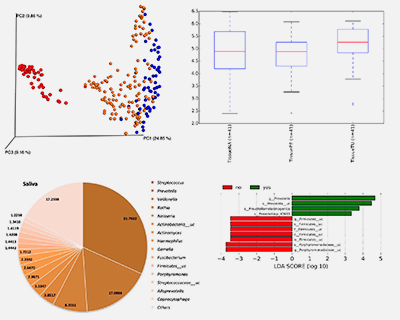 MicroHNSCC
MicroHNSCC
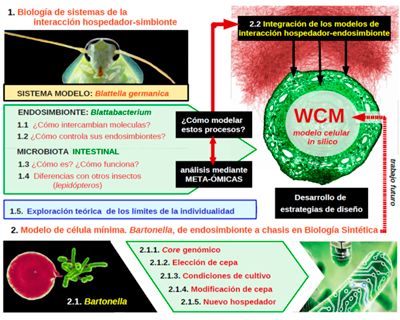
 MODELSYMB
MODELSYMB
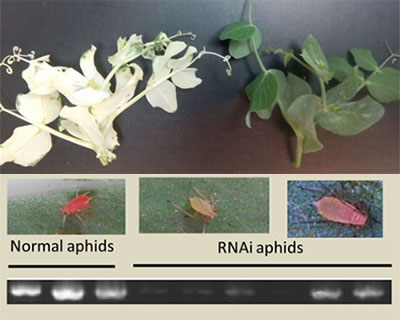 FUNGENAPHID
FUNGENAPHID
 eGUT
eGUT
 STOP
STOP
 CINCHRON
CINCHRON
 GECONAMBRO
GECONAMBRO
 GREEN
GREEN
 InGEMICS
InGEMICS
 CONCISE
CONCISE
 MICROLYNCH
MICROLYNCH
 MICROEVO
MICROEVO
 STABIOME
STABIOME
 SimbVención
SimbVención
 Grupo 2 CIBEResp
Grupo 2 CIBEResp
 ENTOMOPLAST
ENTOMOPLAST
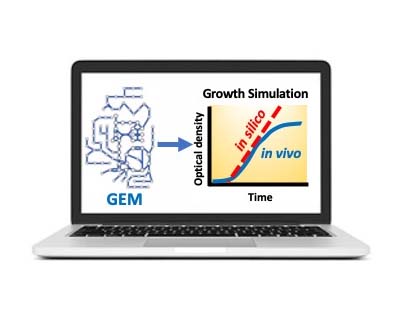 BactGem
BactGem
 MIPLACE
MIPLACE
 SETH
SETH
 IBER-XYFAS
IBER-XYFAS
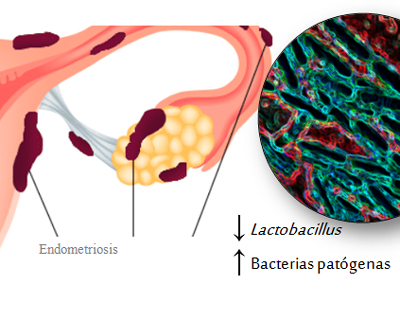 PROFERTILITY
PROFERTILITY
 BIOROBOOST
BIOROBOOST
 NutOxWineYeast
NutOxWineYeast
 HELIOS
HELIOS
 MICROBIOSOL
MICROBIOSOL
 SINNBIOSIS
SINNBIOSIS