The objectives of this platform will be the identification of mutations associated with epidemiological/clinical changes of SARS-CoV-2 with special attention to breakthrough infections.
We are part of a joint effort to better understand the effects of mutations in SARS-CoV-2 in real time. This grant aims at building a platform to deal with current and future threats.
We are part of a joint effort to establish a platform for the screening of antivirals against current and future viral threats. The work combines groups that span the full drug discovery and development process.
The group of Dr. Mar Siles Lucas at IRNASA handles in vitro and in vivo models of parasitic-host interactions with Fasciola hepatica, which could be used to evaluate the potential of the parasite and its molecules to modulate relevant entry routes and inflammation routes in COVID-19. On one hand, this parasite has shown influence on the expression of endocytosis-related molecules (e.g. clathrins) on in vitro mouse epithelial cells, routes that may be relevant for SARS-Cov-2 entry to human cells. In addition, F. hepatica results in a modified Th2 response without the inflammatory component in its host in vivo, and this modulation may result in a controlled inflammatory response to COVID-19.
This collaboration will employ a multi-disciplinary approach to identify inhibitors of SARS-CoV-2 receptor binding and entry. Combining expertise in medicinal chemistry, computational chemical biology, structural biology, and virology, 3 parallel approaches will be employed: (1) Immediate testing of compound libraries (>1,400) using a high-throughput cell assay for SARS-CoV-2 receptor binding and entry. (2) Computational screening and design, synthesis, and evaluation (3). Development of protein-based inhibitors derived from the ACE2 receptor. The final evaluation of hits for effectiveness and drug resistance will be done with SARS-CoV-2.
The project proposes a new strategy for the screening of drugs and antibodies against the SARS-CoV-2 coronavirus. This is an innovative technology for drug screening that uses an affordable, fast, safe and efficient system to evaluate all types of antiviral compounds and antibodies that block the entry of SARS-CoV-2 virus into human cells. The use of recombinant viruses has been successfully used in drug screening for various medically important viruses, as well as in serological screening. The advantages of the proposal are well founded, highlighting that this technology may be applicable in the future to other viruses that constitute an emerging public health problem.
The ability to resolve populations structure at high resolution and in high depth is key for understand viral evolution and pathogenesis. In this project, we are implementing an extreme fidelity next-generation based sequencing techniques to be able to address key questions in viral evolution and pathogenesis.
The main goal of this project is to define the effect of all possible mutations in a viral capsid, and to understand how different cellular and environmental pressures can alter the viability of such mutations in the capsid.
Cellular molecular chaperones are a group of conserved and abundant proteins that oversee protein folding and help maintain protein homeostasis. The goal of this project is to define all the chaperones and co-chaperones involved in the replication of respiratory syncytial virus, the single most important respiratory pathogen in children.
The main aim of this project was to define all the Hsp90 co-chaperones involved in the replication of respiratory syncytial virus, the single most important respiratory infection in infants.

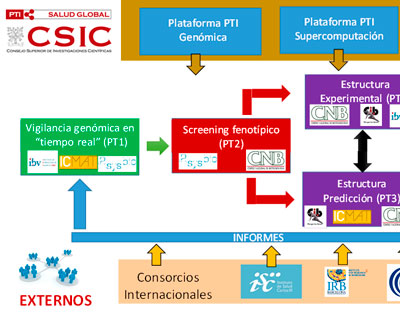 Genomic monitoring
Genomic monitoring
 Mutaciones
Mutaciones
 Antiviral platform
Antiviral platform
 COVID-19: anti-infectious and anti-inflammatory action of immunomodulatory parasite molecules in a safe-to-use synthetic format
COVID-19: anti-infectious and anti-inflammatory action of immunomodulatory parasite molecules in a safe-to-use synthetic format
 BlockAce
BlockAce
 ANTICOR
ANTICOR
 AICO
AICO
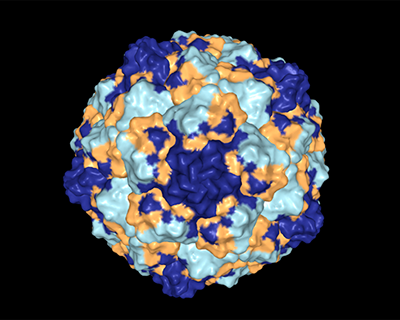 Determining the viable sequence space of a viral capsid
Determining the viable sequence space of a viral capsid
 Chaperone networks in viral respiratory infections
Chaperone networks in viral respiratory infections
 Defining the role of Hsp90 co-chaperones in RSV replication
Defining the role of Hsp90 co-chaperones in RSV replication